
Cell pausing method for adipose tissue derived mesenchymal stem cells: comparison of Petaka G3 and ordinary flask
Introduction
Mesenchymal stem cells (MSCs) have been used in various clinical trials, and most have showed promising results (1-4). We have developed a simple washing, isolation and propagation protocol to produce adipose tissue derived (AT) MSC using platelet lysate supplementation (5,6). Sometimes our AT-MSC culture grows a little faster or slower than usual, so that harvest time does not match with the time when the cells are needed. Moreover, sometimes cell therapy schedule is postponed due to various causes such as: the patient or cell therapy facility is not ready, while cells in culture are already confluent and should be harvested. If confluent cells are not harvested and kept in culture, they will be over confluent. The cells in over confluent culture either tend to differentiate so that they are not stem cells anymore, or undergo aging so that they are not effective in cell therapy (7). Therefore, proliferation of MSCs in ready to harvest culture should be halted by “cell pausing” method, so that they do not increase in number and do not experience senescence. Various methods of cell pausing were applied to prevent proliferation of some cell lines (8), and stem cells, which either required special medium (9,10), using complicated encapsulation method (11), or using special flask (Petaka G3) (12). Therefore this study aimed to develop an easy and economical method of MSC pausing method for our AT- MSCs using ordinary basal medium and flask that yielded AT-MSCs, which were compliant with the requirements of International society for cell therapy (ISCT) (13).
Methods
This was an experimental analytic study on cell pausing of adipose tissue derived MSCs, which was conducted in Stem Cell Medical Technology Integrated Service Unit, Dr. Cipto Mangunkusumo General Hospital/Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia, and Stem Cell and Tissue Engineering (SCTE) Research Center Indonesia Medical Education and Research Institute (IMERI), Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia, from January to July 2019. This study got ethical approval from Ethics Committee Faculty of Medicine Universitas Indonesia (No. ND-609/UN2.F1/ETIK/PPM.00.02/2019).
Sample and procedure
The sample was cryopreserved AT-MSCs (P3) that was stored in SCTE-IMERI. The sample was thawed and cultured in three ordinary T75 flasks until a sufficient number was achieved. Harvested cells were checked for their surface markers, and differentiation potentials, to ensure they meet ISCT guidelines (13). The harvested cells were seeded in four Petaka G3 flasks (double sided, 375,000 cells per side, and 24 mL complete medium), and 20 ordinary T75 flasks (375,000 cells per flask and 10 mL complete medium). The flasks were cultured at 37 °C and 5% CO2, until 70–80% confluent. Then, two Petaka G3 and ten ordinary T75 flasks were put in ±4 °C, and the rest at room temperature (±24 °C).
On day-3, -4, -5, -6, and -7, two T75 flasks were observed, photographed, and harvested, and on day-7 two Petaka G3 flasks were observed, photographed, and harvested. Before harvest, the supernatant was checked for its pH. Harvested cells were checked for viable cell number (trypan blue exclusion method), viability percentage, surface markers (CD73, CD90, CD105, and lineage negative cocktail) and differentiation potentials. Differentiation potential assays were done on 70–80% confluent cultures. Microphotographs of differentiation potential assay intended cultures were taken on day-2, to get insight of cell attachment after cell pausing. Differentiation to chondrogenic lineage was done using prolonged culture method (14) (duplo), and to osteogenic, and adipogenic lineages were done using induction medium (Stempro) (duplo). Cell number and viability was checked twice, before and after washing. The time that was needed to reach 70–80% confluent culture in differentiation potential assays was noted.
Data collection and analysis
Data collected were harvested cell number and viability before and after washing, percentage of CC73, CD90, CD105, and lineage negative, and differentiation potential. The data were presented descriptively as means and standard deviations in graphs. In addition, the time to reach 70–80% confluent culture for cell pausing at 4 °C and room temperature, in T75 and Petaka G3 flasks was noted, and presented descriptively as means and standard deviations.
Statistical analysis (t test) were done to see differences in viable cell number, viability percentages, CD73, CD90, CD105, and CD negative percentages between T75 and Petaka G3 flasks at day-7.
Results
The whole process of cell seeding, culturing and harvesting in T75 was much easier compared to Petaka G3 flasks. The pH of supernatant was more or less stable (Table 1). The cells at day three to seven at cell pausing in T75 as well as Petaka G3 flasks at room temperature showed normal attached spindle shaped morphology, while those at 4 °C showed rounded morphology, and some detached cells were observed at Petaka G3 flasks (Figure 1).
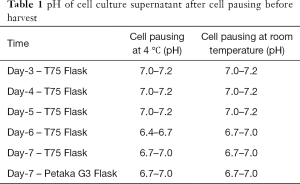
Full table

When cell pausing was done at room temperature, viability percentages before and after centrifugation were more than 90% for both T75 and Petaka G3 flasks. However, when cell pausing was done at 4 °C the viability percentages before centrifugation dropped for both T75 and Petaka G3 flasks, but still higher than 80%, while after centrifugation the viability percentage dropped further especially for T75 flasks, but still higher than 70%. There were no statistical differences in cell viabilities at 4 °C and room temperature between T75 and Petaka G3 flasks at day-7 (Figure 2A).
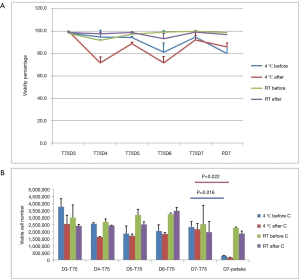
Viable cell number tended to decrease over time when cell pausing was done at 4 °C, and the lowest yield was from Petaka G3 flasks at day-7. There were statistical differences in viable cell numbers at 4 °C between T75 and Petaka G3 flasks, both before and after centrifugation where P=0.016 and 0.022 respectively (Figure 2B). However, when cell pausing was done at room temperature, viable cell number tended to be stable until day-6 and dropped slightly at day-7 for T75 flasks, while viable cell number at day-7 for Petaka G3 flasks was similar to those of T75 flasks (Figure 2B).
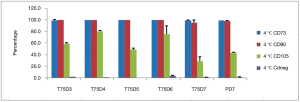
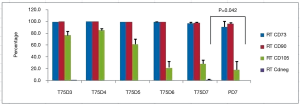
Cell characteristics after cell pausing at both 4 °C and room temperature did not change for CD90, and CD73 for both T75 and Petaka G3 flasks (Figures 3,4). However, CD105 tended to decrease over time to below 30% at day-6 and day-7 for cell pausing at room temperature in T75 and Petaka G3 flasks (Figure 4), while for cell pausing at 4 °C, decrease of CD 105 below 30% only occurred at day-7 in T75 flasks (Figure 3). After cell pausing at room temperature, negative marker did not change in both T75 and Petaka G3 flasks, while at 4 °C, negative markers only increased above 2% in T75 flasks at day-6. There was no statistical difference in cell characteristics between T75 and Petaka G3 flasks at 4 °C and room temperature, except for CD negative at room temperature (P=0.042) (Figure 4). Moreover, after cell pausing at 4 °C and room temperature in both T75 and Petaka G3 flask, the cells could differentiate into three lineages, namely osteogenic, chondrogenic and adipogenic lineages.
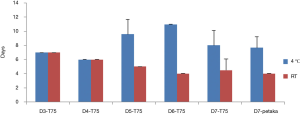
After cell pausing, time to 70–80% confluent can be seen in Figure 5. Cell pausing at room temperature did not cause an increase in time to 70–80% confluent, but cell pausing at 4 °C tended to cause an increase in time to 70–80% confluent. There was no statistical difference in time to 70–80% confluent between T75 and Petaka G3 flasks. This finding is supported by photomicrograph of seeding day-2 (Figure 6). At day-2 after seeding only few cells were attached after cell pausing at 4 °C, while many more were attached after cell pausing at room temperature (Figure 6).

Discussion
Our results showed that cell pausing at room temperature was better than 4 °C in both T75 and Petaka G3 flasks, which was proven by preservation of fibroblastic morphology at room temperature, while at 4 °C the cells were rounded and ready to detach, especially in Petaka G3 flasks, where some cells were detached (Figure 1). Moreover, cell viability was better in cell pausing at room temperature compared to at 4 °C, which at room temperature until day-7 viabilities before and after centrifugation were all above 90% (Figure 2). These findings are in line with the result of Hunt et al (15), which showed that cell pausing of mammalian cell lines at room temperature was better than at 4 °C. In addition, our study showed that cell viability after cell pausing at room temperature were higher than the results of a cell pausing study on cell lines (CHOK1, HEK293, and 1321N1) at 23 °C for four days, which showed that cell viabilities were all above 80% (8). Our cell pausing study showed better results supposedly due to use of fully filled and sealed T75 and Petaka G3 flasks, which prevent substantial pH change (Table 1).
Cell pausing at 4 °C was better in T75 compared to Petaka G3, which was showed by cell yield (viable cell number) that dropped considerably in Petaka G3 flasks. This result was in line with the appearance of cell morphology, where some cells in Petaka G3 flasks were detached. Cell pausing at 4 °C seemed to cause injury that might lead to cell death in case of our AT-MSCs, especially at Petaka G3 flasks.
Cell viability percentages dropped the most after centrifugation after cell pausing at 4 °C, but the percentages were still higher than 70% (Figure 2A), thus still could meet the criteria of FDA for cell therapy (16). However, in this study viability was assessed by trypan blue exclusion method, which was the limitation of our study, as trypan blue only distinguished viable from dead cells, and not from cells that were in the process of dying (early apoptosis) (17). This fact had an impact on functional cell number in cell pausing at 4 °C, which were showed by rounded cell morphology (Figure 1), longer time that was needed to reach 70–80% confluent after cell pausing at day-5 onward (Figure 5), and fewer cell attachments when cell pausing at 4 °C was harvested and re-cultured (Figure 6).
In this study, cell viability in MSC cell pausing at 4 °C in both T75 and Petaka G3 flasks could be maintain above 70% until day-7, which was better compared to storage of harvested cells at 4 °C (18,19). A study on harvested AT-MSC storage at 4 °C showed that storage in physiologic saline and basal medium only could preserve the viability for two days and one day respectively (18). Another study on harvested umbilical cord (UC)-MSC storage at 4 °C showed that storage in high glucose basal medium and physiologic saline could preserve viability for four days, while phosphate buffered saline could preserve viability for three days (19).
In term of cell characteristics, our study showed that CD73, and CD90 could meet the criteria of ISCT for both cell pausing at 4 °C and room temperature, using T75 and Petaka G3 flasks. However, CD105 was reduced gradually during cell pausing at room temperature, while at 4 °C CD105 showed inconsistent pattern of reduction in T75 flasks, where at day-4, CD105 was 80.1% (Figure 3). Cell pausing at 4 °C day-7 showed that CD105 percentage in Petaka G3 was greatly reduced, but was higher than in T75 flask.
Decrease in CD105 (endoglin) cell population is often link to decrease in chondrogenic differentiation potential, however a recent study showed that CD105 expression on MSCs did not predict their chondrogenic differentiation potential (20), which was similar to our result. In addition, decrease in CD105 cell population was apparently not because of the use of different culture flaks (T75 or Petaka G3). It was rather caused by the depletion of serum in the medium during cell pausing (21), as in this study there was no medium change before cell pausing. Moreover, decrease in CD105 might influence the capacity of MSCs to promote vascular dependent cell/tissue regeneration, such as regeneration of cardiac muscle (21), as CD105 has a role in angiogenesis, but decrease in CD105 population might be overcome by adding TGFβ in the medium and culturing in hypoxic condition (21). Changes in the characteristic of cells provide an insight that during cell pausing, modifications of culture medium by adding certain factors need to be conducted in the future, which depend on the intended application of the MSCs.
At room temperature CD negative percentage was significantly lower in Petaka G3 compared to T75 flasks (P=0.042) (Figure 4). However, both percentages were below 2%, thus still met the requirement for cell therapy that was set by ISCT (13).
Cell pausing at room temperature showed that T75 and Petaka G3 flasks gave comparable results in cell morphology, cell viability percentage, viable cell number yield, cell characteristics, and differentiation and attachment capacity. Therefore, cell pausing using T75 is preferable to Petaka G3 due to its easy handling as well as from economical point of view. Therefore this method will very useful in cell therapy, where cells are ready for harvest while patients or cell application facility is not ready or when the cells need to be transported to remote areas that need a certain time to arrive at application site. However, the slim shape of Petaka G3 flasks is more convenient for storage and sending to remote areas, and may provide lower sending cost compared to sending bulky T75 flasks.
In conclusion, the results of cell pausing at room temperature using fully filled and sealed ordinary flasks were comparable to Petaka G3 flasks.
Acknowledgments
Funding: This study was funded by a research grant from the Ministry of Research, Technology and Higher Education of the Republic of Indonesia, Hibah Penelitian Pengembangan 2019, contract no.NKB-1804/UN2.R3.1/HKP.05.00/2019. The authors would like to gratefully acknowledge Mr. Aaron Huebner from Celartia Ltd, who gave the Petaka G3 flasks for this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study got ethical approval from Ethics Committee Faculty of Medicine Universitas Indonesia (No. ND-609/UN2.F1/ETIK/PPM.00.02/2019).
References
- Pawitan JA, Yang Z, Wu YN, et al. Towards Standardized Stem Cell Therapy in Type 2 Diabetes Mellitus: A Systematic Review. Curr Stem Cell Res Ther 2018;13:476-88. [Crossref] [PubMed]
- Freitag J, Bates D, Wickham J, et al. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: a randomized controlled trial. Regen Med 2019;14:213-30. [Crossref] [PubMed]
- Hanley PJ, Lowdell M. Advancing cellular therapies towards standard of care: a focus on testing of cellular therapy products. Cytotherapy 2019;21:275-7. [Crossref] [PubMed]
- Saeedi P, Halabian R, Fooladi AAI. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig 2019;6:34. [Crossref] [PubMed]
- Pawitan JA, Liem IK, Suryani D, et al. Simple lipoaspirate washing using a coffee filter. Asian Biomedicine 2013;7:333-8.
- Suryani D, Pawitan JA, Lilianty J, et al. Comparison of FBS and PRP containing medium effects on human lipoaspirate-derived mesenchymal stem cell proliferation. Med J Indones 2013;22:146-51. [Crossref]
- Turinetto V, Vitale E, Giachino C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int J Mol Sci 2016;17. [Crossref] [PubMed]
- Wise H, Abel PW, Cawkil D. Use of Reduced Temperature Cell Pausing to Enhance Flexibility of Cell-Based Assays. J Biomol Screen 2009;14:716-22. [Crossref] [PubMed]
- Robinson NJ, Picken A, Coopman K. Low temperature cell pausing: an alternative short-term preservation method for use in cell therapies including stem cell applications. Biotechnol Lett 2014;36:201-9. [Crossref] [PubMed]
- Ginis I, Grinblat B, Shirvan MH. Evaluation of bone marrow-derived mesenchymal stem cells after cryopreservation and hypothermic storage in clinically safe medium. Tissue Eng Part C Methods 2012;18:453-63. [Crossref] [PubMed]
- Chen B, Wright B, Sahoo R, et al. A novel alternative to cryopreservation for the short-term storage of stem cells for use in cell therapy using alginate encapsulation. Tissue Eng Part C Methods 2013;19:568-76. [Crossref] [PubMed]
- Celartia. PetakaG3. Available online: http://petaka.com/. Accessed 27 November 2018
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. [Crossref] [PubMed]
- Pawitan JA, Suryani D, Wulandari D, et al. Prolonged culture in FBS and FBS-substitue containing media: Spontaneous chondrogenic differentiation of adipose tissue derived mesenchymal stem cells. Int J PharmTech Res 2014;6:224-35.
- Hunt L, Hacker DL, Grosjean F, et al. Low-temperature pausing of cultivated mammalian cells. Biotechnol Bioeng 2005;89:157-63. [Crossref] [PubMed]
- US FDA. Guidance for FDA reviewers and sponsors: Content and review of chemistry, manufacturing, and control (CMC) information for human somatic cell therapy investigational new drug applications (INDs) (Draft). US FDA; 2008 April. Available online: (Accessed 26 January 2017)http://www.fda.gov/OHRMS/DOC KETS/98fr/03d0349gdl.pdf
- François M, Copland IB, Yuan S, et al. Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy 2012;14:147-52. [Crossref] [PubMed]
- Nofianti CE, Sari IN. Temporary storage solution for adipose derived mesenchymal stem cells. Stem Cell Investig. 2018;5:19. [Crossref] [PubMed]
- Krishnanda SI, Agarwal R, Yausep OE, et al. Comparison of Various Solutions for Temporary Storage of Umbilical Cord Derived Mesenchymal Stem Cells. Annu Res Rev Biol 2017;21:1-8. [Crossref]
- Cleary MA, Narcisi R, Focke K, et al. Expression of CD105 on expanded mesenchymal stem cells does not predict their chondrogenic potential. Osteoarthritis Cartilage 2016;24:868-72. [Crossref] [PubMed]
- Mark P, Kleinsorge M, Gaebel R, et al. Human mesenchymal stem cells display reduced expression of CD105 after culture in serum-free medium. Stem Cells Int 2013;2013:698076. [Crossref] [PubMed]
Cite this article as: Pawitan JA, Liem IK, Kispa TD, Luviah E, Mujadid F, Novialdi . Cell pausing method for adipose tissue derived mesenchymal stem cells: comparison of Petaka G3 and ordinary flask. Stem Cell Investig 2020;7:1.


