Stem Cell Ophthalmology Treatment Study: bone marrow derived stem cells in the treatment of Retinitis Pigmentosa
Introduction
Retinitis Pigmentosa (RP) is a complex, incompletely understood hereditary retinal disorder that interferes with the normal retinol visual pigment cycle causing impairment of rod photoreceptor function primarily. This initially manifests as nyctalopia or difficulty with night vision which is the initial manifestation of impairment of photopigment retinol function and cycling. The photoreceptor impairment leads to irreparable damage and progressive loss of photoreceptors. This initially leads to peripheral vision loss in a ‘ring scotoma’ pattern. As peripheral vision loss accelerates, the vision is confined to a small central area with normal or near normal acuity initially. As photoreceptor loss continues, cone photoreceptors in the macule are lost and the central vision deteriorates more rapidly leading to CF, HM and LP vision. Once sufficient photoreceptors have been lost to reach this level of vision, the visual loss can progress rapidly. Ultimately complete blindness or no light perception (NLP) may result. There are other associated findings in RP including characteristic pigmentary changes in the retina, waxy appearance of the optic disc, posterior subcapsular cataracts, cystoid macular edema (CME) and dust-like particles in the vitreous consisting of melanin granules and various cells.
There are at least 60 different genes associated with RP. Inheritance pattern includes Autosomal Dominant, Autosomal Recessive and X-Linked Recessive. Depending on the gene affected, there are hundreds of different potential point mutations leading to an enormous variety of genetic causes for a similar appearing disease.
Various retinal degenerations including RP affect between approximately 1 in 2,000 (1) and 1 in 3,000 people in the world (2). Various explanations have been postulated including disruption of proteins important to phototransduction, synthesis, structural organization and tertiary folding of rhodopsin. Rhodopsin gene mutations (3) may account for up to 40% of RP in autosomal dominant cases. These may include overexpression of rhodopsin or shortened rhodopsin causing photoreceptor degeneration (4).
Epigenetics has more recently been shown to play an important role in many diseases including ocular disease. The expression or access to genes, such as through methylation, and therefore production of variable proteins may play a key role in RP. An increase in Histone deacetylases (HDAC) activity was seen in a murine model of RP before photoreceptor deterioration (5). Although there are variable roles, HDAC activity may be important for suppression of chromatin access (6). Valproic Acid is an HDAC inhibitor and has been studied for treating patients with RP, although further controlled studies are needed to fully assess any potential benefit (7).
Efforts to mitigate the progressive visual loss in RP have previously been disappointing. Therapy with 15,000 IU/day of vitamin A palmitate has been reported to slow the progression of RP in terms of ERG results, not visual acuity or visual field (8). Docosahexaenoic acid (DHA) therapy was found to slow the decline in visual field sensitivity although there was no effect on the course of the disease (9). Lutein supplementation demonstrated increased macular pigment in approximately 50% of patients with RP but without a change in central visual acuity (10). Hyperbaric oxygen therapy over a 10-year period was shown to slow the expected loss of vision by about 23% but did not halt the loss or increase visual acuity for patients (11).
Retinal prosthesis such as the Argus II Retinal Prosthesis System use a glasses mounted camera that transmits information wirelessly to an epiretinal electrical device that stimulates the inner nerve layers directly (12). This does not replace the lost photoreceptors, however survival of some bipolar and ganglion cells even after severe photoreceptor loss allows for their direct electrical stimulation as an alternative path for visual information to be sent to the brain. It is designed for patients who are totally blind (NLP) or who have bare LP from RP.
The SCOTS NIH identifier NCT 01920867 and the Stem Cell Ophthalmology Treatment Study II (SCOTS 2) Identifier NCT 03011541 are the largest ophthalmology stem cell studies registered with the National Institutes of Health (NIH)–www.clinicaltrials.gov. Both are Institutional Review Board (IRB) approved and utilizes autologous bone marrow derived stem cells (BMSC) in the treatment of optic nerve and retinal diseases.
SCOTS is an open label, non-randomized, efficacy study. There is no placebo or sham arm. All patients meeting eligibility criteria and enrolled in the study receive active treatment. Bone Marrow aspirated from the posterior Iliac Crest is separated to provide BMSC within the stem cell concentrate.
The treatment protocols in SCOTS have been continued in the SCOTS 2.
Inclusion criteria for SCOTS provide that patients:
- Have objective, documented damage to the retina or optic nerve unlikely to improve OR have objective, documented damage to the retina or optic nerve that is progressive AND have less than or equal to 20/40 best corrected central visual acuity in one or both eyes AND/OR an abnormal visual field in one or both eyes;
- Be at least 3 months post-surgical treatment intended to treat any ophthalmologic disease and be stable;
- If under current medical therapy (pharmacologic treatment) for a retinal or optic nerve disease be considered stable on that treatment and unlikely to have visual function improvement (for example, glaucoma with intraocular pressure stable on topical medications but visual field damage);
- Have the potential for improvement with BMSC treatment and be at minimal risk of any potential harm from the procedure;
- Be 18 years of age or older;
- Be medically stable and able to be medically cleared by their primary care physician or a licensed primary care practitioner for the procedure. Medical clearance means that in the estimation of the primary care practitioner, the patient can reasonably be expected to undergo the procedure without significant medical risk to health.
Exclusion criteria include:
- Patients who are not capable of an adequate ophthalmologic examination or evaluation to document the pathology;
- Patients who are not capable or not willing to undergo follow up eye exams with the Principal Investigator or their ophthalmologist or optometrist as outlined in the protocol;
- Patients who are not capable of providing informed consent;
- Patients who may be at significant risk to general health or to the eyes and visual function should they undergo the procedure;
- Presence of concurrent untreated or unstable ocular disease;
- Patient unwillingness to sign the informed consent form;
- Patient inability to adhere to prescribed behavior including avoidance of smoking.
There are three arms of SCOTS with the type of treatment chosen based on the degree of visual loss, etiology of visual loss, associated risk factors for the treatment arms and the patient’s medical risk status. Bilateral treatment is provided assuming both eyes meet eligibility requirements. As these are autologous stem cells, no immunosuppression is required.
An FDA cleared Class 2 medical device is used to separate the bone marrow aspirate into a stem cell concentrate. This concentrate has averaged 1.2 billion Total Nucleated Cells including mesenchymal stem cells in approximately 14–15 cm3 of concentrate. Retrobulbar injection consists of 3 cm3 of concentrate; subtenons injection of 1 cm3; intravitreal injection of 0.05 cm3; subretinal injection of approximately 0.1 cm3 and intra-optic nerve injection of approximately 0.1 cm3. The intravenous injection is provided from the remainder of the concentrate.
Arm 1 consists of stem cell concentrate injected retrobulbar and subtenons followed by intravenous infusion. Patients with ophthalmic conditions that preclude safe or effective utilization of intravitreal injection of concentrate, such as the presence of silicone oil, may be offered Arm 1 if meeting inclusion criteria.
Arm 2 consists of the administration of retrobulbar, subtenon and intravitreal concentrate followed by intravenous infusion. Patients meeting inclusion criteria with visual acuity between 20/40 and 20/200 in one or both eyes and/or visual field loss may be offered Arm 2.
Arm 3 is reserved for retinal and optic nerve patients with severe visual loss (visual acuity of 20/200 or worse in at least one eye). Typically patients admitted to Arm 3 have poorer vision (less than 20/200). Arm 3 consists of the better seeing eye receiving the same treatment as Arm 1 or more typically, Arm 2, and the eye with more extensive visual acuity loss receiving a core pars plana vitrectomy with injection of subretinal or intra-optic nerve concentrate followed by the infusion of intravenous stem cells. Monocular patients are not eligible for Arm 3.
Follow up examinations are required at 1, 3, 6 and 12 months post treatment with reporting of the eye exam results to the Principal Investigator and Study Director.
Methods
The study and data accumulation were carried out with approval from NIH and OHPR compliant IRB, informed consent for the research was obtained from each participating patient, and the study is in accordance with HIPAA regulations.
All patients enrolled in this study underwent a comprehensive ophthalmologic examination, including significant past medical and ocular history, best-corrected Snellen and ETDRS visual acuities, anterior segment biomicroscopy, measurement of intraocular pressure and dilated fundus examination. Automated perimetry (dependent on vision), ocular coherence tomography (OCT) and fundus photography were performed. If there was a suspicion of neovascularization, a fluorescein angiogram was done. The approximate duration of visual loss was obtained from the patient and from a review of the patient’s medical records.
The visual acuity for patients with less than 20/400 on the Snellen chart (i.e., inability to see largest projected Snellen optotype) was measured using a 20/200 “E” card held at varying premeasured distances until the patient reported visualization (the optotype was held in front of the patient but eccentric gaze was permitted). In reporting visions in eyes less than 20/1,000, Count Fingers was recorded. During follow up exams practitioners without a 20/200 “E” card were permitted to directly report Count Fingers. Vision was judged to be hand motions if the hand-held optotype could not be visualized at 1 foot but the patient could perceive hand motions. Patients with ETDRS visual acuities less than 5/200 were checked for the ability to perceive hand motions. Patients who could only perceive light or not perceive light were recorded as “light perception” or “no light perception” respectively.
Each participating patient underwent an extensive discussion in which the experimental nature of the proposed surgery was stressed. All surgeries were performed in an out-patient ambulatory surgery center by one of the authors (JNW).
Examinations were performed the day before surgery and after surgery at 1 and 3 days by one of the authors (JNW). As the patients came from distant geographical locations, postoperative examinations at 1, 3, 6, and 12 months were performed by the patient’s local eye physician. Patients were included in this report if they or their eye physician had provided follow up eye exams with visions which included at least the 6-month post-treatment exam.
In this presentation, results of 17 patients with a clinical diagnosis of RP treated in SCOTS and with adequate post-procedure follow up are reported. Patients had the diagnosis of RP made by their ophthalmologist(s) and confirmed on pre-operative eye exam by Dr. Weiss. Post-procedure exams performed by their ophthalmologist(s) and forwarded for review were required to extend at least to 6 months post-procedure.
Results
The average age of the patients treated was 48.8 years. The average duration of disease prior to treatment was 27.6 years and ranged from 4 to approximately 60 years. Two patients with a history of onset of RP in ‘childhood’ were estimated to have been diagnosed at age 10. The preoperative visual acuity ranged from LP to 20/30 with severe or almost extinguished visual fields. Postoperatively, the visual acuity was LP to 20/30.
Binocular visual acuity using Snellen line equivalents of LogMAR vision was used to assess overall patient results. Individual eyes were evaluated in the same fashion. Eyes with HM or CF vision were converted to Snellen lines of vision equivalents. Per this formula HM is considered 20/20,000, decimal 0.001 and LogMAR 3.0; CF at 2 feet is considered 20/2,000, decimal 0.01, LogMAR 2.0. (13). This conversion is derived from the LogMAR scale which is the log of the minimum angular resolution. Individual eyes were assessed using Snellen acuity including converting CF and HM to Snellen equivalents which ranged from 20/800 to 20/1,600 (14,15). Patients with acuity of less than CF at 4 Feet or with reports of vision from follow up exams that reported CF without a specific distance, were classified as CF vision of 2 feet and considered a Snellen acuity of 20/2,000, decimal 0.01, LogMAR 2.0. All visions of CF at 4 Feet or better were reported as Snellen equivalents with concomitant decimal and LogMAR visions including extrapolation of LogMAR for CF 6 feet and CF 4 feet. LP vision was not reported as LogMAR. For calculations of lines of vision and percentage improvements or loss, additional individual letters (the + or – recorded in acuity) were not utilized. In calculating the percentage of change in improved eyes, the delta or difference between the LogMAR pre-procedure acuity and post- procedure acuity was divided by the pre-procedure acuity to compare the amount of improvement to baseline vision. This can be written as Pre LogMAR-Post LogMAR/Pre LogMAR (Table 1).
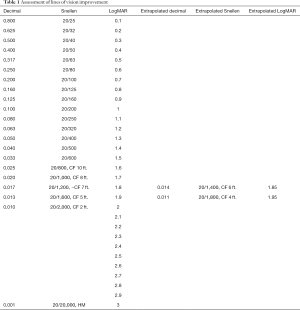
Full table
In this study, with the exception of 1 patient with NLP vision in one eye, both eyes were treated (33 eyes). There were no intraoperative or postoperative surgical complications. A total of 17 patients with RP who were able to provide data at least including the 6-month visit are included in the report.
Eleven of the 17 patients (64.7%) showed improved binocular vision averaging 10.23 lines of Snellen acuity per eye over pre-treatment acuity; 8 patients (35.3%) remaining stable over the follow up period in this otherwise progressive disease and no patients experiencing loss of overall acuity. There was an average of 31% improvement in the vision of patients with pre-operative vision of at least HM or greater.
In 33 treated eyes, 15 eyes (45.5%) improved an average of 7.9 Snellen lines of vision, 15 eyes (45.5%) remained stable, and 3 (9%) worsened by an average of 1.7 lines of Snellen acuity. Improvements ranged from 1 to 27 lines of vision. Using the Logmar Scale and calculating delta as a ratio to pre-treatment vision in improved eyes, acuity improvement over pre-treatment vision ranged from 23% to 90% with an average of 40.9% visual acuity improvement over baseline vision. Evaluation of all eyes capable of LogMAR vision showed an average of 31% improvement in vision over baseline.
Statistical evaluation determined P=0.016 and was determined with the Wilcoxon Sign Ranked Test. Eyes that were LP did not show any discernable change in acuity.
A summary of the data is presented in Table 2.
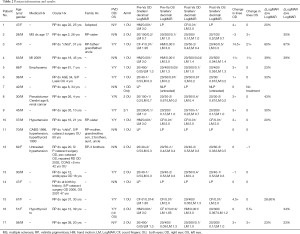
Full table
Patient #3, who reported the acute worsening of visual acuity OU several weeks before the SCOTS procedure, obtained the most improvement in visual acuity following surgery. This may imply that the treatment may have provided a “rescue” prior to irretrievable visual loss.
The presence/absence of a PVD did not appear significant. There did not appear to be a significant difference in the effectiveness of the Arm 1 versus the Arm 2 procedure.
Discussion
Although positive outcomes in RP have been shown in the SCOTS clinical trial, it would be reasonable to assume that this procedure may be more effective for some, but not all, genetic mutations. It is a limitation of this study that with rare exception, the patients had not undergone genetic testing. In addition, regulation of gene expression includes a wide array of mechanisms that cells use to up or downregulate gene products. Modification of gene transcription, methylation, histone acetylation, post-transcriptional regulation including micro RNA (miRNA) and regulation of translation may all play roles in the expression of abnormal genes and affect responses to the use of BMSC.
Another study limitation was that after the immediate postoperative period, examinations were performed by the patient’s local eye physician who did not typically have access to ETDRS visual acuities. Patients who came from distance returned to their local eye physician for follow-up. Having the postoperative examinations performed by an eye physician, unassociated with SCOTS, was felt to help eliminate any potential bias. Although ETDRS visual acuities were taken preoperatively by the Principal Investigator, they were excluded since there were no postoperative comparisons.
It is likely the initial nyctalopia experienced by patients with RP reflects the impairment of photoreceptor function rather than significant loss of photoreceptors. The early maintenance of visual acuity and peripheral vision may be a consequence of sufficient replacement of lost photoreceptors by endogenous retinal stem cells. The gradual deterioration of vision in RP may therefore reflect the depletion of these stem cells and subsequent decrease or absence of replacement of the damaged photoreceptors, inner plexiform and ganglion cell layers. BMSC, specifically CD34+ cells provided by intravitreal injection in SCOTS have been confirmed on immunofluorescence microscopy to undergo neuronal transdifferentiation into Neuronal Nuclei (NeuN) positive cells (16). Their migration into the inner neuronal layers and potentially to the outer nuclear and photoreceptor layers with transdifferentiation into photoreceptors or bipolar cells may be a partial explanation of their effectiveness. In addition, BMSC have been shown to release exosomes including those containing miRNA, as well as Nerve Growth Factor (NGF), Brain Derived Neurotrophic Factor (BDNF), Ciliary Body Neurotrophic Factor (CBNF) and other neurotrophic factors that may be stimulatory to residual bipolar and photoreceptor cells (17). Mitochondrial transfer may also play a role as these may be depleted in these highly metabolically active cells (18). Methylation of cytosine is a key epigenetic factor regulating gene expression, as well as cell survival, and has been detected in deteriorating RP photoreceptors in murine models; inhibition of DNA methyltransferases (DNMTs) has been shown to reduce photoreceptor death in an RP model retinal explant (19). We postulate that the presence of BMSC may affect epigenetic properties of the photoreceptor cells as well as neurons in the inner and ganglion cell layers, potentially improving their function and recovery through access to compensatory genes.
Autologous BMSC have the same genetic sequences as all cells in an individual. The rationale for using BMSC to treat genetic conditions in patients with progressive visual disabling conditions is that the stem cells may re-set the ocular condition to an earlier time when the patient had better vision. We have previously published that the SCOTS treatment of 5 patients with Leber’s Hereditary Optic Neuropathy showed meaningful visual improvement maintained over a period of months (20). Researchers have reported vision related quality of life improvements in RP following intravitreal BMSC. Although not specifically reporting vision, the improvement in lifestyle was positive although appeared to diminish with time (21). Given that these conditions are hereditary and progressive, a patient may experience a worsening of vision subsequent to the procedure. Potential retreatment may again result in improvement and a return of vision to an earlier time in the disease course. Most recently the authors published statistically positive data on a degenerative condition affecting the optic nerve called Non-arteritic Ischemic Optic Neuropathy (NAION) with 80% of patients achieving binocular improvement and 73.6% of eyes gaining vision (P=0.019) (22). It appears that retinal and neurologic tissue can respond positively to BMSC intervention.
Prior to the use of BMSC in the SCOTS clinical studies, it has been difficult to identify treatment approaches capable of improving or maintaining visual acuity for patients with RP. Using autologous BMSC in accordance with the SCOTS protocol, we have demonstrated that meaningful visual improvement is possible in patients with this otherwise progressive and blinding condition. It is hoped that further research will confirm these findings.
Acknowledgements
The authors would like to acknowledge the statistical evaluation of our data provided by Professor Rosemary Milewski Danaher, MS, MBA of Sacred Heart University and Fairfield University, member ASA.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The procedures were in accordance with the ethical standards of the responsible committee on human experimentation (IRB00002637). All appropriate patient consent for publication of this work has been obtained.
References
- Sohocki MM, Daiger SP, Bowne SJ, et al. Prevalence of mutations causing retinitis pigmentosa and other inherited retinopathies. Hum Mutat 2001;17:42-51. [Crossref] [PubMed]
- Sullivan LS, Daiger SP. Inherited retinal degeneration: exceptional genetic and clinical heterogeneity. Mol Med Today 1996;2:380-6. [Crossref] [PubMed]
- Dryja TP, McGee TL, Hahn LB, et al. Mutations within the rhodopsin gene in patients with autosomal dominant retinitis pigmentosa. N Engl J Med 1990;323:1302-7. [Crossref] [PubMed]
- Lee ES, Flannery JG. Transport of truncated rhodopsin and its effects on rod function and degeneration. Invest Ophthalmol Vis Sci 2007;48:2868-76. [Crossref] [PubMed]
- Sancho-Pelluz J, Alavi MV, Sahaboglu A, et al. Excessive HDAC activation is critical for neurodegeneration in the rd1 mouse. Cell Death Dis 2010;1:e24. [Crossref] [PubMed]
- Delcuve GP, Khan DH, Davie JR. Roles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitors. Clin Epigenetics 2012;4:5. [Crossref] [PubMed]
- Clemson CM, Tzekov R, Krebs M, et al. Therapeutic potential of valproic acid for retinitis pigmentosa. Br J Ophthalmol 2011;95:89-93. [Crossref] [PubMed]
- Berson EL, Rosner B, Sandberg MA, et al. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol 1993;111:761-72. [Crossref] [PubMed]
- Berson EL, Rosner B, Sandberg MA, et al. Clinical trial of docosahexaenoic acid in patients with retinitis pigmentosa receiving vitamin A treatment. Arch Ophthalmol 2004;122:1297-305. [Crossref] [PubMed]
- Aleman TS, Duncan JL, Bieber ML, et al. Macular pigment and lutein supplementation in retinitis pigmentosa and Usher syndrome. Invest Ophthalmol Vis Sci 2001;42:1873-81. [PubMed]
- Vingolo EM, Rocco M, Grenga P, et al. Slowing the degenerative process, long lasting effect of hyperbaric oxygen therapy in retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol 2008;246:93-8. [Crossref] [PubMed]
- Ahuja AK, Dorn JD, Caspi A, et al. Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. Br J Ophthalmol 2011;95:539-43. [Crossref] [PubMed]
- Holladay JT. Proper method for calculating average visual acuity. J Refract Surg 1997;13:388-91. [PubMed]
- Watt WS. How Visual Acuity is Measured. Eye Conditions 2003. Available online: http://lowvision.preventblindness.org/eye-conditions/how-visual-acuity-is-measured/
- Schulze-Bonsel K, Feltgen N, Burau H, et al. Visual acuities "hand motion" and "counting fingers" can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci 2006;47:1236-40. [Crossref] [PubMed]
- Kim JY, You YS, Kim SH, et al. Epiretinal membrane formation after intravitreal autologous stem cell implantation in a retinitis pigmentosa patient. Retin Cases Brief Rep 2017;11:227-31. [Crossref] [PubMed]
- Wilkins A, Kemp K, Ginty M, et al. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res 2009;3:63-70. [Crossref] [PubMed]
- Liu K, Ji K, Guo L, et al. Mesenchymal stem cells rescue injured endothelial cells in an in vitro ischemia-reperfusion model via tunneling nanotube like structure-mediated mitochondrial transfer. Microvasc Res 2014;92:10-8. [Crossref] [PubMed]
- Farinelli P, Perera A, Arango-Gonzalez B, et al. DNA methylation and differential gene regulation in photoreceptor cell death. Cell Death Dis 2014;5:e1558. [Crossref] [PubMed]
- Weiss JN, Levy S, Benes SC. Stem Cell Ophthalmology Treatment Study (SCOTS): bone marrow-derived stem cells in the treatment of Leber's hereditary optic neuropathy. Neural Regen Res 2016;11:1685-94. [Crossref] [PubMed]
- Siqueira RC, Messias A, Messias K, et al. Quality of life in patients with retinitis pigmentosa submitted to intravitreal use of bone marrow-derived stem cells (Reticell -clinical trial). Stem Cell Res Ther 2015;6:29. [Crossref] [PubMed]
- Weiss JN, Levy S, Benes SC. Stem Cell Ophthalmology Treatment Study: bone marrow derived stem cells in the treatment of non-arteritic ischemic optic neuropathy (NAION). Stem Cell Investig 2017;4:94. [Crossref] [PubMed]
Cite this article as: Weiss JN, Levy S. Stem Cell Ophthalmology Treatment Study: bone marrow derived stem cells in the treatment of Retinitis Pigmentosa. Stem Cell Investig 2018;5:18.


