Stem cell extracellular vesicles and kidney injury
Introduction
The acute and chronic loss of renal function are interrelated syndromes with a rising incidence as well as poor treatment outcomes and high-related costs. Acute kidney injury (AKI) is characterized by a rapid decline of renal function and associates with an increase of mortality and hospitalization. In fact, AKI is a common complication in hospitalized patients with acute illness thus having great impact on public health resources (1). Moreover, 8% to 16% of patients with AKI develop chronic renal failure (2). Chronic kidney disease (CKD) may progress to end-stage renal disease (ESRD), resulting from a maladaptive response to injury, with fibrosis and progressive loss of function. In developed countries, diabetic nephropathy (DN) is one of the main causes of ESRD due to escalation in the frequency of obesity and diabetes (3). The main therapies for loss of renal function include dialysis and kidney transplantation (4). None of them is satisfactory and the increase of survival rate after therapies is not adequate. While hemodialysis has high cost and elevated complications, the number of compatible kidney organs is not enough to satisfy the organ need (5). For these reasons, there is an urgent need to find new effective therapeutic strategies.
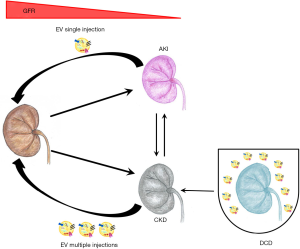
In the last decades, many reports demonstrated the improvement of renal dysfunction by the administration of stem cells (4). In particular, mesenchymal stromal cells (MSCs) have been extensively used in experimental models of kidney injury (6) as well as in clinical trials (4,7). The described mechanism of action is mainly due to the release of trophic factors including growth factors, cytokines and extracellular vesicles (EVs), favoring tissue repair and reducing inflammation (8). Recently, literature data highlight the use of stem cell-derived EVs per se as innovative option, alternative to cell based strategies, to treat renal failure in pre-clinical models (9). In fact, EVs released by different types of stem cells are described to ameliorate AKI and to prevent chronic progression in several preclinical experimental models (Tables 1 and 2) (34). EVs may influence different cell types acting on physiological processes such as proliferation (35), angiogenesis (36) and immune escape (9). In the present review, we intend to offer an update on the pre-clinical models used to test EVs in prevention and treatment of renal injury, as well as in pre-transplant conditioning, and on the mechanisms involved (Figure 1).
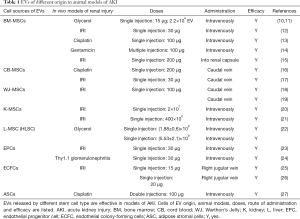
Full table
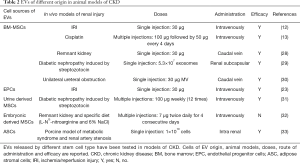
Full table
Stem cell EVs and AKI
EVs are a heterogeneous population of double layers membrane fragments, differing for size, sedimentation rate and floating density (37,38). They are released by most of cell types and contain several biologically active molecules such as proteins, lipids and nucleic acids (mRNAs and non-coding RNAs such as miRNAs and other small RNAs) (39). EVs may influence recipient cells by various mechanisms such as surface receptor interaction, direct stimulation or by transfer of proteins and/or genetic material (40-43). Due to the common characteristics and pro-regenerative potential of MSCs of several organs, MSC-derived EVs of different origin have been tested in preclinical models (44). In particular, EVs have been obtained from bone marrow MSCs (BM-MSCs), from adipose derived MSCs (ASCs) and from fetal MSCs: cord blood (CB) and Warthon’s Jelly (WJ), as well as from resident MSCs isolated from renal or liver tissues (45,46). Recently, MSCs from urine were also isolated and applied in experimental models of renal injury (47).
MSC-EVs and AKI
The most common feature of AKI is the rapid loss of renal tubular cells with a decrease of renal function and increase in retention markers such as blood urea nitrogen and creatinine (48). There are different experimental models to mimic AKI in animals, performed preferentially in rodents. The triggering event may be a toxic compound, an ischemic insult or a metabolic dysfunction (7).
The first evidence of the beneficial effect of EVs shed by BM-MSCs was proved by Bruno et al. in 2009 (10), in a model of AKI induced by glycerol injection in SCID mice. In this model, intramuscle injection of glycerol causes rhabdomyolysis, with a detrimental effect on kidney function that picks between day 1 and 3 post injection (10,49). The authors showed that a single intravenous administration of EVs at the peak of damage accelerated morphological and functional recovery (10). The effect of EVs was superimposable to that induced by the originating MSCs suggesting that EVs may substitute cell treatment. Moreover, EVs purified by a liver resident MSCs (HLSCs) were intravenously administered in the AKI model induced by glycerol and improved renal functionality and morphology (22).
BM-MSC EVs were also tested in AKI lethal models induced by toxic drugs. The cisplatin nephropathy is a lethal or sub-lethal model of AKI, depending on the dose used, characterized by a rapid loss of renal function with a pick 4 days post drug treatment (50,51). A single EV administration increased the survival of SCID mice (13). In the same model, EVs obtained by CB-MSCs resulted effective by protecting from oxidative stress, stimulating cell proliferation and reducing cell apoptosis (16). Similarly, EV derived from BM-MSCs abated renal dysfunction in an AKI model caused by gentamycin injection (14). In all toxic models, EVs reduced the renal histological lesions such as presence of luminal cell debris and tubular hyaline casts as well as necrosis of proximal and distal tubular cells (13,14,16).
Another common feature of different types of AKI is hypoxia, frequently associated with oxidative stress response (52). Ischemia/reperfusion injury (IRI) model, used to mimic hypoxic insult, is usually obtained by a renal artery and vein occlusion of one or both kidneys, followed by the reestablishment of oxygen supply (53). In order to evaluate whether EVs could be effective on the protection by ischemic damage, EVs were administered intravenously immediately after the transitory deficiency of the blood flow in rats (12,17-19). EVs released by BM-MSCs resulted effective (12). The same positive effect was obtained using EVs released by MSCs derived from fetal tissues such as CB-MSCs (17) and by WJ-MSCs (18,19) with different mechanisms of action. The injection of EVs shed by CB-MSCs accelerated tubular cells dedifferentiation and growth via rat HGF induction and human HGF transfer. On the contrary, EVs released by WJ-MSCs have been described to be able to stimulate proliferation and to reduce inflammation and apoptosis via mitochondrial protection (18,19). Moreover, in a murine model of IRI, BM-MSC EVs were injected under the renal capsule resulting effective mainly by suppressing inflammation (15). In addition, the effect of EVs released by renal MSCs were also investigated (20,21). Ranghino et al. demonstrated that MSCs isolated from glomeruli and their EVs recovered kidney function and reduced ischemic damage stimulating tubular cell proliferation (21). In the same model, the administration of a population of a resident intratubular CD133 positive, with characteristics of progenitor cells, contributed to renal recovery (54). However, no effect was observed by use of the CD133 positive cell-derived EVs (21). In addition, it has been shown that EVs released by mouse renal MSCs cultured under hypoxia were able to stimulate angiogenesis in vitro and to improve peritubular microvascular rarefaction in vivo (20).
Altogether, these data indicate a potent effect of single EV-MSC administration obtained from different sources in all models of AKI tested (Table 1). The mechanisms appear multiple, promoting proliferation and survival of resident cells and limiting inflammation, oxidation and vessel rarefaction.
Endothelial progenitor-derived EVs and AKI
Human endothelial progenitors cells (EPCs) are a population of progenitors with strong angiogenic ability that circulate in peripheral blood and characterized by the expression of a variety of vascular surface markers. The EVs (EPC EVs) released by EPC isolated from healthy subjects have been tested as pro regenerative factors to improve renal function. In particular, injection of EPC EVs immediately after IRI prevented renal functional injury (23) by stimulation of tubular cell proliferation, reduction of inflammation and apoptosis (23). In addition, EPC EVs resulted effective in a rat model that mimics human mesangial proliferative glomerulonephritis obtained by intravenous injection of anti-Thy1.1 antibody. Treatment with EPC EVs reduced proteinuria, mesangial cells damage and glomerular infiltration of inflammatory cells, improving renal functionality (24). EVs have been also purified by an alternative source of pro-angiogenic endothelial colony-forming cells (ECFCs). ECFCs derive from human umbilical CB and are endothelial precursors with high angiogenic and proliferative capacities (25). The efficacy of ECFC EVs was tested in an AKI model induced by IRI resulting in the inhibition of macrophage infiltration, oxidative stress and tubular necrosis (25). The same group better investigated the key factors contained within EVs responsible for the beneficial effect (26). They showed that miR-486-5p carried by EVs was transferred to renal injured tissue, decreasing PTEN and activating AKT with a reduction of the ischemic injury (26).
Effect of EV subpopulations in AKI
Strategies used to isolate EVs from cell supernatants include polymer-based methods, gel-permeation chromatography, membrane filtration, affinity capture, density-gradient centrifugations and ultracentrifugation. Among the heterogenic EV populations, a special effort was dedicated to identify the active fraction/s in the promotion of kidney regeneration. Regardless the protocol adopted to isolate EVs, the main result consists on separation by size or density into the two EV main fractions: exosomes and microvesicles. These two fractions are distinct by site of generation and size. Exosomes are released from multivesicular intracellular bodies with a smaller size (around 30–100 nm), while microvesicles derive from cell surface budding and have a size up to 500 nm (37). Furthermore, the use of gradient floating allows separating in a major number of EV fractions based on their density. Recently Collino et al. by discontinuous density gradient separation analyzed three BM-MSC EV fractions characterized by the differential expression of typical exosomal markers. These fractions showed differential pro-proliferative and anti-apoptotic capabilities in vitro (55).
For example, murine monocrotaline pulmonary hypertension model treated with MSC-derived exosomes prevented and reversed pulmonary hypertensive changes typical of the damage. Conversely the treatment with MSC-derived microvesicles was not able to protect from the pulmonary hypertension, underling the different functional properties of the two EV types (56).
The functional difference between EV fractions has been also studied in in vivo AKI models (9). Recently, Bruno et al. showed how BM-MSC exosomes obtained by differential ultracentrifugation clearly improved renal function and morphology in a murine glycerol-AKI model, comparable to total EVs. On the contrary, the microvesicles were not able to induce the same positive effect (57). Accordingly, Burger et al. demonstrated that exosomes and microvesicles isolated by ultracentrifugation from ECFCs promoted a different effect on IRI induced endothelial cell injury. Only the treatment with ECFC-conditioned media or exosomes completely blocked apoptosis, while the microvesicles were ineffective. The same result was observed in murine model of AKI induced by IRI where the treatment with ECFC-derived exosomes significantly attenuated renal injury (25).
Contrary to all previous studies, Wen et al. demonstrated that total MSC EVs were more effective in the protection of bone marrow cells after irradiation than microvesicles or exosomes per se (58). These works underline the requirement of further studies providing data on the functional analysis of single EV fractions due to their possible different abilities.
Stem cell EVs and CKD
CKD is a complex pathology, which progressively occurs within time and differs for causes, severity and rate of progression in response to a number of possible inducers (7). The pre-clinical investigation of the efficacy of EVs in CKD therefore evaluated not only the different cell sources for EV isolation but also different doses, number and timing of administration (Table 2). Not less important is the starting point for treatment, that may be in the early stages to prevent progression of CKD, or when the disease is well established to revert chronic features.
Several experimental models are available to mimic the broad range of pathologies included in the classification of human CKD. One of the main drivers of human CKD is diabetes. The early key features of DN are podocyte damage/loss and mesangial cell hypertrophy followed by an increase of extracellular matrix protein deposition (59). The first report of the beneficial effect of EVs in DN was very recently described by Jiang et al., in a rat model of diabetes inducted by streptozotocin injection. EVs isolated from MSCs derived from urine were administered weekly by intravenous injection of 100 µg of EVs for 12 weeks from the onset of diabetes. They were effective in prevention of DN progression (31). EVs induced a reduction of urine volume and urinary micro albumin excretion as well as a protection of podocyte and tubular epithelial cells from apoptosis. Analyses of EV content revealed the presence of protective factors such as transforming growth factor-β1, angiogenin and bone morphogenetic protein-7 (31). In addition, in both models of type 2 diabetes induced by high fatty diet and type 1 diabetes caused by streptozotocin, the effect of stem cells per se (BM-MSCs) was compared with that of conditioned medium (CM) (29). MSC and MSC-CM therapies promoted a regeneration of injured kidney tissue suppressing cell infiltration and reducing interstitial fibrosis and glomerular alteration (29). They also demonstrated that key factors in the biological activity of cells were exosomes by their injection under the renal capsule in the streptozotocin DN model. One administration of exosomes generated a rapid improvement of renal morphology observed one or two weeks after EV treatment (29). Moreover, in a porcine model of metabolic syndrome and renal artery stenosis, a single intrarenal administration of EVs derived from autologous ASCs attenuated renal inflammation and improved medullary oxygenation and fibrosis four weeks post treatment. IL10 was demonstrated as the main driver of the positive effect as vesicles with pre-silenced IL10 were ineffective (33).
Another in vivo model of CKD consists in a surgical five-sixth resection of the kidney tissue, also called remnant kidney model. It is characterized by a rapid decrease of nephron and glomerular filtration rate with glomerular hypertension that leads to glomerulosclerosis and fibrosis (60). He and co-workers showed that multiple injections of BM-MSC derived EVs prevented renal failure in the five-sixth model (28). The same others showed effect of BM-MSC derived EVs in a model of renal fibrosis induced by obstruction of the ureter (30). In tubular cells in vitro, EV treatment abrogated the morphological changes and the increase of the α-SMA secretion induced by transforming growth factor-β1 stimulation (30).
In a similar model of five-sixth resection combined with L-NG-nitroarginine and 6% NaCl diet, multiple administrations of CM purified from human embryonic MSCs (twice daily intravenously for four consecutive days), after the development of CKD attenuated the deterioration of renal function. Six weeks after treatment, glomerular filtration rate and effective renal plasma flow were restored (32). On the contrary, exosomes isolated from the same cells resulted ineffective (32). This is the only paper, to our knowledge, that proposes the use of EVs in an established model of CKD. At variance, in the other models described here, EVs were administered immediately after damage in a preventive approach (12,17,18).
In addition, several studies also indicate a beneficial effect of EVs in the progression toward fibrosis after AKI (12,13,23). In addition, in experimental models of renal IRI, EPC EVs limited the progression from AKI to CKD with a beneficial effect both on kidney tubular epithelial cells and on peritubular endothelial cells (23).
Mechanism of action
In recent years, many efforts were done, through RNA sequencing and proteomic analyses, to fully characterize RNA species (mRNAs or miRNAs) and proteins enriched in EVs and possibly involved in the biological activities observed (61). The recovery of AKI by EVs has been ascribed to several molecules that induced phenotypic changes in renal cells. MiRNAs are probable candidates for cell reprogramming toward a pro-regenerative phenotype (11). Indeed, many studies show that RNAs carried by EVs are the pivotal mechanism for their therapeutic function (62). The relevant role of miRNAs in the recovery of AKI was well demonstrated by non-specific miRNA depletion by Dicer or Drosha knockdown in stem cells (9,11,23). Drosha-knockdown cells generated EVs similar for surface molecule expression from those of native cells but ineffective in in vivo AKI model (11). To better elucidate the mechanism of action of MSC EVs and AKI, de Almeida et al. identified a group of miRNAs carried by mouse ASC EVs that targeted mRNAs associated with Wnt/TGF-β, fibrosis, and epithelial–mesenchymal transition signalling pathways (27). Authors demonstrated that miR-880, miR-141, miR-377, and miR-21 participated on regenerative process in vivo in an AKI model induced by cisplatin (27).
In addition, silencing of specific pro-angiogenic miRNAs (miRNA-126 and miRNA-296) in EPC EVs showed their relevant role in renal regeneration by abrogating EV efficacy in vivo after pro-angiogenic miRNAs depletion (23). These results indicate that miRNA cargo contributes to a reprogramming of renal cells toward regeneration.
The protein content of EVs was also extensively studied in the last years, especially that of EVs released by MSCs (63-65). They contained proteins that are connected to many biological aspects such as angiogenesis, extracellular matrix remodelling, regulation of inflammation, cell cycle and proliferation, cell migration and morphogenesis (63-65). Altogether, these results highlight the complex nature and the multiple effects of EVs that possibly may act in concert to exert their beneficial effects.
Renal biodistribution of EVs
Several reports demonstrated that the stem cell biological activities do not require cell localization within damaged organs (66). At variance, the transfer of EV cargo within damaged target cells is the first requirement for their biological function. EVs express several adhesion molecules involved in their up-take into renal tubular epithelium (10,67). Their ligand-receptor interaction seems fundamental also in vivo. In fact, pre-treatment of EVs with trypsin, that disrupts surface molecules, abolished EVs in vivo effect (10).
In physiological condition, intravenously injected EVs do not accumulate within kidney tissue not are cleared into urine, and spleen and liver are the main organs of localization (68). However, during damage, increase of permeability and cell loss favor EV tissue localization via peritubular capillaries or glomerular passage. By ex vivo immunofluorescence, MSC EVs and EPC EVs were localized within peritubular capillaries and tubules, immediately after their injection (one hour) in AKI models. The maximal accumulation was observed after six hours (10,12,23). Similar results were obtained by in vivo optical imaging technique: labelled MSC EVs using fluorescent lipophilic dyes localized within the renal tissue in an AKI model (9,68). The possibility to image and follow EV biodistribution in vivo may help to understand their regenerative potential. Different approaches of EV labelling such as fluorescent protein-based imaging, luciferase enzymatic activity and super paramagnetic iron oxide nanoparticles have not been tested yet in models of renal disease (68,69).
Stem cell EVs and kidney transplant
The ever-increasing demand for kidneys available for transplantation leads to take in account the kidney donation after circulatory death (DCD) as possible option to limit organ shortage. The use of hypothermic machine perfusion is currently used to ameliorate the viability of DCD kidneys. As MSCs injected in a renal transplant model are able to reduce IRI and to protect the graft (70), Gregorini et al. proposed a new application of MSCs: their use in pre-transplant graft perfusion. In this study, the pre-conditioning with MSCs or MSC-derived EVs in a rat model of DCD kidney was compared to standard perfusion solution in a hypothermic machine perfusion (71). DCD kidneys treated with MSC-derived EVs showed a significant lower global renal damage level than control kidneys. The observed effect of EVs was superior to MSCs. In fact, in MSC-perfused kidneys damage progression was only limited in the early stages, whereas in MSC-derived EV-perfused kidneys the development of ischemic damage was effectively stopped (71). Next step will consists in the characterization of factors contained in EVs that play an important role in the reduction of damage to promote the transplant of kidneys pre-conditioned with MSCs or MSC EVs. The application of these emerging therapies could be evaluated in organs not only from DCD donors but also from brain death donors included in the category “extended criteria donors” in accordance to Crystal City criteria (72). Moreover, an additional use of EVs in kidney transplantation could be envisaged by their systemic administration during transplant. This is supported by a recent report showing that EPC EVs i.v. injected at the moment of transplantation enhanced neoangiogenesis of human islets xenotransplanted in SCID mice, thus improving engraftment and function (73).
Conclusions
In conclusion, EVs appear a promising approach for renal regeneration (Figure 1). The beneficial effects of EVs, especially those derive from MSCs, are well established for the repair of AKI in several experimental models of renal injury. On the contrary, due to the complexity of the pathology, the application of EVs should be deeper investigated in CKD to better define therapeutic doses and the schedule of administration. Nevertheless, some evidence based on pre-clinical models, suggest the potential use of EVs also in a chronic damage. A promising new approach is the use of EVs for conditioning of kidneys before transplant. In addition, a further perspective for the application of EVs could be their engineering to potentiate their therapeutic cargo, by modification of surface molecules or by increasing active molecules (proteins or RNAs).
Acknowledgements
B Bussolati received grant support from Unicyte AG.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pozzoli S, Simonini M, Manunta P. Predicting acute kidney injury: current status and future challenges. J Nephrol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Rewa O, Bagshaw SM. Acute Kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 2014;10:193-207. [Crossref] [PubMed]
- Campion CG, Sanchez-Ferras O, Batchu SN. Potential Role of Serum and Urinary Biomarkers in Diagnosis and Prognosis of Diabetic Nephropathy. Can J Kidney Health Dis 2017;4:2054358117705371. [Crossref] [PubMed]
- Roushandeh AM, Bahadori M, Roudkenar MH. Mesenchymal Stem Cell-based Therapy as a New Horizon for Kidney Injuries. Arch Med Res 2017;48:133-46. [Crossref] [PubMed]
- Yeo WS, Zhang YC. Bioengineering in renal transplantation: technological advances and novel options. Pediatr Nephrol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Wang Y, He J, Pei X, et al. Systematic review and meta-analysis of mesenchymal stem/stromal cells therapy for impaired renal function in small animal models. Nephrology (Carlton) 2013;18:201-8. [Crossref] [PubMed]
- Tetta C, Weiss S, Grange C, et al. Adult Stem Cells and Extracellular Vesicles in Acute and Chronic Kidney Injury. Current Regenerative Medicine 2016;6:2-15. [Crossref]
- Cantaluppi V, Biancone L, Quercia A, et al. Rationale of mesenchymal stem cell therapy in kidney injury. Am J Kidney Dis 2013;61:300-9. [Crossref] [PubMed]
- Bruno S, Camussi G. Exploring mesenchymal stem cell-derived extracellular vesicles in acute kidney injury. Methods Mol Biol 2014;1213:139-45. [Crossref] [PubMed]
- Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol 2009;20:1053-67. [Crossref] [PubMed]
- Collino F, Bruno S, Incarnato D, et al. Camussi. AKI recovery induced by mesenchymal stromal cell-derived extracellular vesicles carrying microRNA. J Am Soc Nephrol 2015;26:2349-60. [Crossref] [PubMed]
- Gatti S, Bruno S, Deregibus MC, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant 2011;26:1474-83. [Crossref] [PubMed]
- Bruno S, Grange C, Collino F, et al. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS One 2012;7:e33115. [Crossref] [PubMed]
- Reis LA, Borges FT, Simões MJ, et al. Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One 2012;7:e44092. [Crossref] [PubMed]
- Shen B, Liu J, Zhang F, et al. CCR2 Positive Exosome Released by Mesenchymal Stem Cells Suppresses Macrophage Functions and Alleviates Ischemia/Reperfusion-Induced Renal Injury. Stem Cells Int 2016;2016:1240301. [Crossref] [PubMed]
- Zhou Y, Xu H, Xu W, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther 2013;4:34. [Crossref] [PubMed]
- Ju GQ, Cheng J, Zhong L, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS One 2015;10:e0121534. [Crossref] [PubMed]
- Zou X, Zhang G, Cheng Z, et al. Microvesicles derived from human Wharton's Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther 2014;5:40. [Crossref] [PubMed]
- Gu D, Zou X, Ju G, et al. Mesenchymal Stromal Cells Derived Extracellular Vesicles Ameliorate Acute Renal Ischemia Reperfusion Injury by Inhibition of Mitochondrial Fission through miR-30. Stem Cells Int 2016;2016:2093940. [Crossref] [PubMed]
- Choi HY, Moon SJ, Ratliff BB, et al. Microparticles from kidney-derived mesenchymal stem cells act as carriers of proangiogenic signals and contribute to recovery from acute kidney injury. PLoS One 2014;9:e87853. [Crossref] [PubMed]
- Ranghino A, Bruno S, Bussolati B, et al. The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury. Stem Cell Res Ther 2017;8:24. [Crossref] [PubMed]
- Herrera Sanchez MB, Bruno S, Grange C, et al. Human liver stem cells and derived extracellular vesicles improve recovery in a murine model of acute kidney injury. Stem Cell Res Ther 2014;5:124. [Crossref] [PubMed]
- Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int 2012;82:412-27. [Crossref] [PubMed]
- Cantaluppi V, Medica D, Mannari C, et al. Endothelial progenitor cell-derived extracellular vesicles protect from complement-mediated mesangial injury in experimental anti-Thy1.1 glomerulonephritis. Nephrol Dial Transplant 2015;30:410-22. [Crossref] [PubMed]
- Burger D, Viñas JL, Akbari S, et al. Human Endothelial Colony-Forming Cells Protect against Acute Kidney Injury Role of Exosomes. Am J Pathol 2015;185:2309-23. [Crossref] [PubMed]
- Viñas JL, Burger D, Zimpelmann J, et al. Transfer of microRNA-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney Int 2016;90:1238-50. [Crossref] [PubMed]
- de Almeida DC, Bassi ÊJ, Azevedo H, et al. A Regulatory miRNA-mRNA Network Is Associated with Tissue Repair Induced by Mesenchymal Stromal Cells in Acute Kidney Injury. Front Immunol 2017;7:645. [Crossref] [PubMed]
- He J, Wang Y, Sun S, et al. Bone marrow stem cells-derived microvesicles protect against renal injury in the mouse remnant kidney model. Nephrology (Carlton) 2012;17:493-500. [Crossref] [PubMed]
- Nagaishi K, Mizue Y, Chikenji T, et al. Mesenchymal stem cell therapy ameliorates diabetic nephropathy via the paracrine effect of renal trophic factors including exosomes. Sci Rep 2016;6:34842. [Crossref] [PubMed]
- He J, Wang Y, Lu X, et al. Micro-vesicles derived from bone marrow stem cells protect the kidney both in vivo and in vitro by microRNA-dependent repairing. Nephrology (Carlton) 2015;20:591-600. [Crossref] [PubMed]
- Jiang ZZ, Liu YM, Niu X, et al. Exosomes secreted by human urine-derived stem cells could prevent kidney complications from type I diabetes in rats. Stem Cell Res Ther 2016;7:24. [Crossref] [PubMed]
- van Koppen A, Joles JA, van Balkom BW, et al. Human embryonic mesenchymal stem cell-derived conditioned medium rescues kidney function in rats with established chronic kidney disease. PLoS One 2012;7:e38746. [Crossref] [PubMed]
- Eirin A, Zhu XY, Puranik AS, et al. Mesenchymal stem cell-derived extracellular vesicles attenuate kidney inflammation. Kidney Int 2017;92:114-24. [Crossref] [PubMed]
- Bruno S, Porta S, Bussolati B. Extracellular vesicles in renal tissue damage and regeneration. Eur J Pharmacol 2016;790:83-91. [Crossref] [PubMed]
- Zhan C, Ma CB, Yuan HM, et al. Macrophage-derived microvesicles promote proliferation and migration of Schwann cell on peripheral nerve repair. Biochem Biophys Res Commun 2015;468:343-8. [Crossref] [PubMed]
- Gai C, Carpanetto A, Deregibus MC, et al. Extracellular vesicle-mediated modulation of angiogenesis. Histol Histopathol 2016;31:379-91. [PubMed]
- Lötvall J, Hill AF, Hochberg F, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 2014;3:26913. [Crossref] [PubMed]
- Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol 2015;40:82-8. [Crossref] [PubMed]
- Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2013.2. [PubMed]
- Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cells-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia 2006;20:847-56. [Crossref] [PubMed]
- Ratajczak MZ, Ratajczak J. Horizontal transfer of RNA and proteins between cells by extracellular microvesicles: 14 years later. Clin Transl Med 2016;5:7. [Crossref] [PubMed]
- Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-9. [Crossref] [PubMed]
- Derkus B, Emregul KC, Emregul E. A New Approach in Stem Cell Research-Exosomes: Their Mechanism of Action Via Cellular Pathways. Cell Biol Int 2017;41:466-75. [Crossref] [PubMed]
- Motavaf M, Pakravan K, Babashah S, et al. Therapeutic application of mesenchymal stem cell-derived exosomes: A promising cell-free therapeutic strategy in regenerative medicine. Cell Mol Biol (Noisy-le-grand) 2016;62:74-9. [PubMed]
- Bruno S, Chiabotto G, Camussi G. Concise review: different mesenchymal stromal/stem cell populations reside in the adult kidney. Stem Cells Transl Med 2014;3:1451-5. [Crossref] [PubMed]
- Bruno S, Collino F, Tetta C, et al. Dissecting paracrine effectors for mesenchymal stem cells. Adv Biochem Eng Biotechnol 2013;129:137-52. [Crossref] [PubMed]
- Tian SF, Jiang ZZ, Liu YM, et al. Human urine-derived stem cells contribute to the repair of ischemic acute kidney injury in rats. Mol Med Rep 2017;16:5541-8. [PubMed]
- Heyman SN, Rosenberger C, Rosen S. Acute kidney injury: lessons from experimental models. Contrib Nephrol 2011;169:286-96. [Crossref] [PubMed]
- Grange C, Moggio A, Tapparo M, et al. Protective effect and localization by optical imaging of human renal CD133+ progenitor cells in an acute kidney injury model. Physiol Rep 2014;2:e12009. [Crossref] [PubMed]
- Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 2004;15:1794-804. [Crossref] [PubMed]
- Morigi M, Introna M, Imberti B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells 2008;26:2075-82. [Crossref] [PubMed]
- Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury: from pathophysiology to treatment. J Renal Inj Prev 2015;4:20-7. [PubMed]
- Tögel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation independent mechanisms. Am J Physiol Renal Physiol 2005;289:F31-42. [Crossref] [PubMed]
- Bussolati B, Camussi G. Renal injury: Early apoptotic extracellular vesicles in injury and repair. Nat Rev Nephrol 2017;13:523-4. [Crossref] [PubMed]
- Collino F, Pomatto M, Bruno S, et al. Exosome and microvesicle-enriched fractions isolated from mesenchymal stem cells by gradient separation showed different molecular signatures and functions on renal tubular epithelial cells. Stem Cell Rev 2017;13:226-43. [Crossref] [PubMed]
- Aliotta J M, Pereira M, Wen S, et al. Exosomes induce and reverse monocrotalineinduced pulmonary hypertension in mice. Cardiovasc Res 2016;110:319-30. [Crossref] [PubMed]
- Bruno S, Tapparo M, Collino F, et al. Renal Regenerative Potential of Different Extracellular Vesicle Populations Derived from Bone Marrow Mesenchymal Stromal Cells. Tissue Eng Part A 2017;23:1262-73. [Crossref] [PubMed]
- Wen S, Dooner M, Cheng Y, et al. Mesenchymal stromal cell derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016;30:2221-31. [Crossref] [PubMed]
- Levey AS, Coresh J. Chronic kidney disease. Lancet 2012;379:165-80. [Crossref] [PubMed]
- Heuer JG, Harlan SM, Yang DD, et al. Role of TGF-alpha in the progression of diabetic kidney disease. Am J Physiol Renal Physiol 2017;312:F951-F962. [Crossref] [PubMed]
- Nargesi AA, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for renal repair. Curr Gene Ther 2017;17:29-42. [Crossref] [PubMed]
- György B, Szabó TG, Pásztói M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 2011;68:2667-88. [Crossref] [PubMed]
- Eirin A, Zhu XY, Puranik AS, et al. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci Rep 2016;6:36120. [Crossref] [PubMed]
- Anderson JD, Johansson HJ, Graham CS, et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells 2016;34:601-13. [Crossref] [PubMed]
- Kim HS, Choi DY, Yun SJ, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res 2012;11:839-49. [Crossref] [PubMed]
- Bi B, Schmitt R, Israilova M, et al. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 2007;18:2486-96. [Crossref] [PubMed]
- Lindoso RS, Collino F, Bruno S, et al. Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev 2014;23:1809-19. [Crossref] [PubMed]
- Grange C, Tapparo M, Bruno S, et al. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int J Mol Med 2014;33:1055-63. [PubMed]
- Di Rocco G, Baldari S, Toietta G. Towards Therapeutic Delivery of Extracellular Vesicles: Strategies for In Vivo Tracking and Biodistribution Analysis. Stem Cells Int 2016;2016:5029619. [Crossref] [PubMed]
- Rampino T, Gregorini M, Bosio F, et al. Mesenchymal stromal cells injection reduces acute rejection damage in a rat experimental model of kidney transplantation. G Ital Nefrol 2011;28:132-4. [PubMed]
- Gregorini M, Corradetti V, Pattonieri EF, et al. Perfusion of isolated rat kidney with Mesenchymal Stromal Cells/Extracellular Vesicles prevents ischaemic injury. J Cell Mol Med 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Rosengard BR, Feng S, Alfrey EJ, et al. Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant 2002;2:701-11. [Crossref] [PubMed]
- Cantaluppi V, Biancone L, Figliolini F, et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant 2012;21:1305-20. [Crossref] [PubMed]
Cite this article as: Grange C, Iampietro C, Bussolati B. Stem cell extracellular vesicles and kidney injury. Stem Cell Investig 2017;4:90.


