
Immune reconstitution profile after allogeneic hematopoietic stem cell transplantation with post-transplant cyclophosphamide
Graft-versus-host disease (GVHD) remains a major cause of non-relapse mortality (NRM) after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Traditionally, a combination of calcineurin inhibitor (CNI) and methotrexate (MTX) has been used as GVHD prophylaxis in matched donors. Post-transplant cyclophosphamide (PTCy), by functional impairment of alloreactive T-cell, has also emerged as a highly effective GVHD prophylaxis strategy (1,2). However, the effect of PTCy on post-transplant immune reconstitution (IR) is unclear. Furthermore, whether there is any difference in IR with 1 or 2 doses of PTCy is also not well established. Within this context, we conducted this retrospective study to compare IR profile in peripheral blood HSCT outcomes in 137 patients with and without PTCy at our institution between 2012 and 2016.
We constructed 3 cohorts. Patients undergoing matched related donor (MRD) allo-HSCT with tacrolimus (TAC)/MTX based GVHD prophylaxis (MRD, n=48). Patients undergoing fully matched unrelated donor (MUD) allo-HSCT with 1 dose of PTCy, TAC, and mycophenolate mofetil (MMF) as GVHD prophylaxis (MUD, n=61). Patients undergoing haplo-HSCT with 2 doses of PTCy, TAC and MMF as GVHD prophylaxis (Haplo, n=28). The PTCy dose was 50 mg/kg on day 3 for MUD and on days 3 and 4 for Haplo. There has been an effort to balance between GVHD and graft versus tumor effect by optimization of PTCy. One strategy is using single dose of PTCy instead of two. We have published our phase II clinical trial of 1 dose of PTCy in myeloablative, peripheral blood stem cell, MUD transplantation with effective acute GVHD but only modest chronic GVHD control (these patients are included in the MUD cohort in this analysis) (3). Similarly, in non-myeloablative haploidentical bone marrow transplant, there was a trend towards higher extensive chronic GVHD with 1 dose of PTCy, when compared to 2 doses (2).
Preparative regimens were myeloablative in all cases except 4. IR was evaluated via serial flow cytometry analysis of lymphocytes on days 30, 60, 100, 180, and 365. The following panel was used: CD3 (T-lymphocytes), CD4 (T-lymphocytes), CD8 (T-lymphocytes), CD45 (leukocytes), CD27, CD19 (B-lymphocytes), CD197 [CC-type chemokine receptor 7 (CCR7)], CD16 [natural killer (NK) cells], CD28, CD56 (NK cells) and CD57. Gating strategy is provided in Figure S1 and Table S1.
In our cohort, 49% were males and the median age was 53 (range, 20–71) years. The median times (range) to neutrophil (P=0.001) and platelet (P=0.07) engraftment were 13 [8–16], 12 [9–16], 16 [13–18] and 21 [19–24], 28 [26–37], 34 [32–47] days for MRD, MUD, and Haplo, respectively. There were no graft failures for the entire cohort. The day 100 cumulative incidence (CI) of CMV infection was 30% (95% CI, 25–33%) for MRD, 41% (95% CI, 37–49%) for MUD, and 61% (95% CI, 57–71%) for Haplo (P=0.006). The day 100 CI of BK virus was 7% (95% CI, 5–11%), 12% (95% CI, 10–17%), and 18% (95% CI, 13–22%) for MRD, MUD, and Haplo, respectively. There was no difference in incidence of bacterial or fungal infections between the three cohorts. The day 100 CI of grade III–IV acute GVHD was 12% (95% CI, 8–17%), 15% (95% CI, 13–22%), and 16% (95% CI, 14–26%), respectively. The 2-year CI of severe chronic GVHD was 16% (95% CI, 12–21%) for MRD, 26% (95% CI, 19–32%) for MUD, and 16% (95% CI, 13–23%) for Haplo. One-year overall survival (OS) for the entire cohort was 69% (95% CI, 46–81%) with no significant difference between the cohorts.
Lymphocyte subset recovery (T, B, and NK cells) for MUD and Haplo was significantly less (P=0.02) when compared to MUD at day 30 post-transplant. This difference became statistically insignificant by day 60 and remained so through day 365 (Figure 1A). Recovery of CD4+ naïve T-cells was significantly less through day 180 for Haplo. Recovery of both CD4+ and CD8+ naïve T cells was generally slower for Haplo during the first year as well. Central memory (CD45−, CD27+, CD197+) effector memory (CD45−, CD27−, CD197−) and effector T cell (CD8+, CD3+, CD27−) recovery was equivalent across all cohorts (Figure 1B).
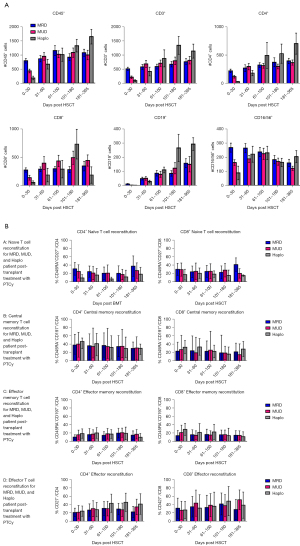
In our report, we find that IR was impaired in PTCy recipients in the immediate post-transplant period but quickly recovered to that seen in patients without PTCy. Furthermore, we did not find a survival difference between patients treated with and without PTCy.
IR has been identified as an important factor affecting not only susceptibility to infections but also survival. Recovery of both innate and adaptive immune compartments is dependent on both peripheral expansions driven by host cytokines and antigenic milieu as well as de-novo production from allogeneic hematopoietic progenitors (4,5). In our cohort we noted the early delay in recovery for haplo-HSCT recipients which denotes the effect of both the donor source as well as the use of PTCy. It is notable that there was also an early delay in IR for MUD (1 dose of PTCy), likely reflecting that this delay in IR is not dose dependent and reflects upon donor type and whether PTCy was administered at all. Donor type has shown to influence IR. Matched donors have yielded better IR outcomes and potential reasons for this include HLA mismatch leading to mixed lymphocyte reactions and host-versus-graft alloreactivity that can delay IR (6). Mehta et al. described IR at day 100 in patients undergoing either MUD, MRD, or Haplo in a cohort where all received PTCy and also noted delayed recovery in CD4+ and CD8+ T cells in MUDs/Haplos when compared to MRDs which again points toward the importance of donor type in IR of T cells (7). Massoud et al. compared IR profile between PTCy and anti-T-lymphocyte globulin (ATLG) and reported faster recovery of CD8+ T and NK cells with ATLG and of CD4+ T and B cells with PTCy. These differences led to a higher infection rate with PTCy, like our CMV findings, but did not translate into a survival advantage for either platform (8).
In a pediatric cohort, a relative sparing of the CD4+ T cell population and suppression of CD8+ population has been noted with PTCy, unlike our cohort, which may reflect lower thymic reserve in our patients due to advanced age (9). While we did not include functional assays in our study, a recent study by Zhao et al. showed that while non-regulatory T (non-Treg) cells were suppressed regardless of whether PTCy was administered, this suppression was deeper in the PTCy group regardless of CD4 or CD8 status. However, while PTCy recipients were able to recovery numerically, both CD4+ and CD8+ compartments had significantly higher expression of inhibitory molecules such as programmed cell death protein 1 (PD-1), T cell immunoreceptor with Ig and ITIM domains (TIGIT), and T cell immunoglobulin mucin-3 (TIM-3), and this was persistent on the CD8+ population up to day 180 with less interferon-gamma (IFNy) production upon anti-CD3/CD28 stimulation. This reflects that PTCy derived suppression of naïve, effector and memory T cells might be due to direct effect or through Treg crosstalk and that while numerical recovery will eventually occur, functional handicap might persist which would explain the lower rates of GVHD and higher rates of infections (10).
In conclusion, our study adds to the existing literature regarding IR profile in PTCy recipients, its relative safety, and the necessity to continue to monitor these patients for long term infectious complications. Functional analysis of T-cell immune phenotype, by investigating inhibitory receptors, of long-term survivors should be performed to clarify whether these qualitative defects in immunity are eventually overcome.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Stem Cell Investigation. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://sci.amegroups.com/article/view/10.21037/sci-2023-002/coif). AS reports royalty fees from In8Bio Inc. and consultant fees from Kite. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Holtan SG, Hamadani M, Wu J, et al. Post-Transplant Cyclophosphamide, Tacrolimus, and Mycophenolate Mofetil As the New Standard for Graft-Versus-Host Disease (GVHD) Prophylaxis in Reduced Intensity Conditioning: Results from Phase III BMT CTN 1703. Blood 2022;140:LBA-4. [Crossref]
- Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008;14:641-50. [Crossref] [PubMed]
- Jamy O, Innis-Shelton R, Bal S, et al. Phase II clinical trial of one dose of post-transplant cyclophosphamide for graft versus host disease prevention following myeloablative, peripheral blood stem cell, matched-unrelated donor transplantation. Am J Hematol 2021;96:E396-8. [Crossref] [PubMed]
- van Den Brink M, Leen AM, Baird K, et al. Enhancing immune reconstitution: from bench to bedside. Biol Blood Marrow Transplant 2013;19:S79-83. [Crossref] [PubMed]
- van den Brink MR, Velardi E, Perales MA. Immune reconstitution following stem cell transplantation. Hematology Am Soc Hematol Educ Program 2015;2015:215-9. [Crossref] [PubMed]
- Elmariah H, Brunstein CG, Bejanyan N. Immune Reconstitution after Haploidentical Donor and Umbilical Cord Blood Allogeneic Hematopoietic Cell Transplantation. Life (Basel) 2021;11:102. [Crossref] [PubMed]
- Mehta RS, Saliba RM, Ghanem S, et al. Haploidentical versus Matched Unrelated versus Matched Sibling Donor Hematopoietic Cell Transplantation with Post-Transplantation Cyclophosphamide. Transplant Cell Ther 2022;28:395.e1-395.e11. [Crossref] [PubMed]
- Massoud R, Gagelmann N, Fritzsche-Friedland U, et al. Comparison of immune reconstitution between anti-T-lymphocyte globulin and posttransplant cyclophosphamide as acute graft-versus-host disease prophylaxis in allogeneic myeloablative peripheral blood stem cell transplantation. Haematologica 2022;107:857-67. [Crossref] [PubMed]
- Sheikh IN, Alqahtani S, Ragoonanan D, et al. Post-Transplant Cyclophosphamide after Matched Sibling and Unrelated Donor Hematopoietic Stem Cell Transplantation in Pediatric Patients with Acute Myeloid Leukemia. Int J Mol Sci 2022;23:8748. [Crossref] [PubMed]
- Zhao C, Bartock M, Jia B, et al. Post-transplant cyclophosphamide alters immune signatures and leads to impaired T cell reconstitution in allogeneic hematopoietic stem cell transplant. J Hematol Oncol 2022;15:64. [Crossref] [PubMed]
Cite this article as: Espinoza-Gutarra M, Saad A, Jamy O. Immune reconstitution profile after allogeneic hematopoietic stem cell transplantation with post-transplant cyclophosphamide. Stem Cell Investig 2023;10:8.


