
Recreating heterogeneity of bladder cancer microenvironment to study its recurrences and progression
Introduction
Recently, Wang et al. published a study using single cell analysis from bladder cancer (BCa) biopsies to identify proteins involved in, what still constitutes one of the major challenges for the treatment of this disease, namely the recurrence of cancer and its progression to an invasive stage (1). The importance of this study lies in to hope that in the near future, the combination of various techniques will make it possible to set up therapeutic strategies that are effective and adapted to individual patients in order to achieve the best possible disease management.
BCa
Among the pathologies that affect humans, cancers represent not only the deadliest forms but also the most delicate to treat, particularly when they are not detected early. Among these, BCa is relatively common and, due to its high potential for recurrence, it entail a significant expense for the health systems of developed countries. Worldwide, 573,000 new cases of BCa have been diagnosed in 2020, with an estimated mortality of 212,000 people (2). While the incidence of BCa is gradually, albeit very slowly, declining in developed countries, its mortality rate has remained about the same for several decades. Two main forms of BCa can be distinguished: the non-invasive form (non-muscle invasive BCa) in 80% to 90% of cases, which can be multifocal, and the invasive form (muscle invasive BCa) which represents 10% to 20% cases and is the deadliest form (3). As it is well stated in Wang et al.’s work, two critical issues of BCa are its high recurrence rate and resistance to treatment.
Current treatments for BCa and its recurrence
The non-invasive BCa forms are treated by transurethral resection bladder tumour (4), where the surgeon removes the tumor masses in the bladder, followed by observation and adjuvant platinum-based chemotherapy or immunotherapy treatments [Bacillus Calmette-Guérin (BCG)] (5). The more serious and invasive BCa forms are treated by a radical cystectomy, which consists in removing the entire bladder but sometimes also the surrounding tissues (6). It should be noted that recurrences after treatment will occur in 80% of non-invasive BCa cases, which can then progress to an invasive form in 30% of patients (7). In the last three decades, no significant improvement resulted from research to reduce the mortality of the BCa. Immunotherapy has recently raised hopes, but the results still seem limited, even if they may in the future constitute one of the weapons for treating patients. Invasive BCa presents a relatively poor response to immunotherapy [26% for anti-programmed cell death-1 or programmed cell death ligand-1 (PD-1/PD-L1) therapy] due to its high rate of mutations in immune microenvironment (8). The modulation of interfering RNAs also seems to be a promising avenue, although with its also limited results (9). Moreover, the global microenvironment of BCa remains poorly understood limiting the occurrence of new therapeutic strategies that would target key elements necessary for tumour progression or recurrences.
The bladder anatomy
The bladder is a hollow organ whose function is urine storage before its evacuation (10,11). From the outer surface to its lumen, the bladder is made up of successive layers of tissue: (I) an adventitia composed of fatty tissue. (II) A thick layer of muscle tissue called the detrusor. It is this muscle that allows the contraction of the bladder to evacuate the urine stored. (III) The lamina propria, a layer of connective tissue that is quite loose and of variable thickness. It accommodates contractions and nourishes the epithelium of the bladder, the urothelium. The lamina propria is the connective tissue populated with fibroblasts, which can be turned into cancer-associated fibroblasts (CAFs) during cancer progression. (IV) The urothelium is made up of three sets of joined cell layers. The basal layer, a single layer of cells, contains stem/progenitor cells, sitting on the basal lamina, essential for the normal renewal of the urothelium. Also connected to the basal lamina, 3 to 5 cell layers of racket-shaped intermediate cells also including few progenitor cells which quickly regenerate the blood/urine barrier during wound healing. Another proportion of the intermediate cells present a higher level of differentiation and replace the superficial cells during normal cell turn-over. These superficial cells constitute the last layer of the urothelium, not connected to the basal lamina, containing the most differentiated cells. In close contact with urine, a toxic liquid waste, these cells are protected, and protect the lower layers, thanks to a layer of specialized proteins: uroplakins. Combined with a close cohesion of the superficial cells ensured by tight junctions, this protein plaque ensures an almost perfect sealing of the epithelial barrier. In the case of cancerous cells, the expression of uroplakins is altered and the superficial layer becomes more permeable, allowing the effective use of treatment by intravesical instillation.
Heterogeneity of BCa cells
Because the mortality of patients with BCa has remained high with no sign of decreasing for several decades, new innovative treatments are needed to combat this disease. It is therefore necessary to identify new therapeutic strategies. The molecular mechanisms behind the initiation and development of BCa and in particular that linked to recurrence remain poorly understood. Indeed, this is made difficult because molecular studies have demonstrated a high heterogeneity of BCa cells, which makes the reproduction of the tumor microenvironment fairly complex (7). The models that currently exist do not make it possible to transcribe this complexity and therefore do not produce results that are transferred in clinical gain (12,13). The development of techniques such as single cell RNA sequencing can help solve, at least in part, the problems related to cellular heterogeneity. It is in this context that the article by Wang et al. represented an interesting avenue. Indeed, this study has made it possible to highlight a subpopulation of cancer cells found in recurrent BCa with an enrichment of the Enhancer of Zeste Homologue (EZH)-2 via, a critical histone methyltransferase that is involved in the epithelial-mesenchymal transition. Heterogeneity is also found in non-cancerous cells in the tumour or in its neighbourhood. Overall, this cellular heterogeneity is reminiscent of what is observed in several other cancer type (14).
CAFs
As it is well highlighted by Wang et al. in their work (1), the fibroblasts present in both normal and tumor tissues form heterogeneous populations. The CAF are an important cell population in tumour, where they can account for 70% of the stromal cells present. They remodeled the extracellular matrix (ECM), created supportive niches for cancer cells, contributed to the tumour metabolism reprogramming, weakened the tumour immune microenvironment and promoted cancer cells invasion in the connective tissues (15,16). Moreover, several sub-types of CAF have been identified, and participate to the heterogeneity of the tumor (16). Alpha smooth muscle actine (αSMA) positive and fibroblast activation protein (FAP) positive CAFs seems to have opposite effects. Whereas αSMA positive CAF depletion leads to poorly differentiated tumours and low survival, FAP positive CAF depletion reduces tumour growth and increases the presence of antitumorigenic lymphocytes (16). Also, the ratio between podoplanin positive and Fibroblast-specific protein-1 positive CAFs, two other markers of CAFs, correlates with clinical outcomes. CAF can be divided in cancer restraining or cancer promoting CAFs (16). While not explored in Wang’s study, spatial heterogeneities have also been reported. For instance, myofibroblasts-like CAFs expressing high levels of αSMA are more like to be found near the tumour margins (17). They are responsible for ECM deposition and potential stiffening of the tumor stroma. More distantly of the tumor, low αSMA-CAFs, called inflammatory CAFs, which promote the proliferation of cancer cells and the recruitment of immune cells. Another CAF subpopulation, the antigen-presenting CAF, have also been identified in pancreatic cancers (18). This shows the importance of clearly defining the different cell populations in or around a tumour, and their evolution in order to better understand the tumour microenvironment.
BCa stem cells
In their work, Wang et al. underscored the heterogeneity of a BCa stem cell population. Cancer stem cells (CSCs) or tumour-initiating cells (TICs) are a subpopulation of cancer cells that can drive tumour initiation and growth and can cause recurrence of the pathology (19). CSCs are resistant to treatment and can modify the tumour environment under the pressure of drugs or radiation. In several cancers, the origin of these cells is the stem cells of the tissue, which share several features with CSC. Notably, CSC have the ability of self-renewal but also to differentiate in various lineages allowing production of heterogeneity in cancer. The proliferative capacity of such cells creates a bias in favor of CSCs after tumor dissociation and cell culture expansion. Several markers for CSC have been identified, including metabolism reprogramming, but they remain limited and not completely specific. Wang et al. suggested that the single cell approach is well suited to explore such questions. However, how all these different and heterogeneous cell populations, including the immune cells which were omitted in Wang’s work, interact together to promote disease progression remains an open question.
Needs for development of new research models
One of the main ongoing issues in the field of oncology is the lack of adequate models to explore and establish causation of information obtained from clinical data in a setting that is truly representative of the disease, resulting in very low clinical translation rate (12,13). This remains the case for the work from Wang and collaborators. While they used a basic xenograft mouse model, their observations remain limited to tumor size, and thus their results on EZH2 in relation to BCa recurrence and eventual resistance to treatment remains mostly correlative. Importantly, implication of EZH2 in tumor progression is not new and has been suggested in numerous tumor types from breast to prostate cancer and it can act as either as a tumor suppressor or as an oncogene (20). A similar observation can be made on the epithelial-to-mesenchymal transition, whereas it constitutes a wide spectrum and cancer cells that present intermediary phenotype might be more metastatic (21-23). Some of those works have relied on the use of engineered experimental 3D models to investigate heterogeneity in a context of a heterogeneous population (21,22). Therefore, given the identified cell subpopulations, a logical next step would be to explore their actual contribution to BCa progression and resistance to therapeutics strategies in more advanced experimental models. This also highlights the need to develop new BCa models capable of faithfully reproducing tumours and with a sufficient scale to carry out a reasonable volume of experiments.
As hinted above, engineered 3D models are increasingly used to mimic different stages of tumor progression (Figure 1). These 3D models are however extremely varied and encompass methods ranging from 3D hydrogel to spheroids composed of a single type of cell and complex fully engineered models reproducing cancerous cells in their environment including cellular, matrix and immune, passing through various types of hydrogels or organoids. While very simple models based on hydrogels, such as collagen, Matrigel or biocompatible materials allow production of a controlled 3D environment, they suffer from limitations resulting from the difference in their composition and organization compared to the native tumour ECM.
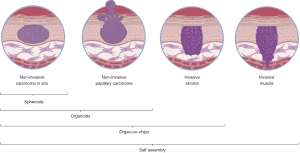
Spheroids and organoids are also interesting options. They do provide a 3D cellular structure that recreates some aspects of the tumor, including the creation of a hypoxic core in the center and a proliferative zone at its periphery. These models can also help when studying the drug penetration and efficiency. Such models can give good indications on tumour organization, especially when several cell types compose them. It is known that E- and N-cadherin expression change depending of the cell culture conditions and then impact research on epithelial-mesenchymal transition (EMT). Spheroids can also be combined with 3D structures such as hydrogels or various types of more elaborate matrices to form more complex models. Interestingly, patient derived BCa organoids have been successfully established before (24), which makes them a prime model candidate to investigate the role of cell heterogeneity in BCa.
The state-of-the-art tissue engineered models, such as organ-on-a-chip, tissue bioprinting or self-assembly (25), offer the most future possibilities to perform advanced validation of clinical or basic science data. Organ-on-chips are microfluidic devices used for mimicking the physiology of organs (26). They constitute an alternative to more complex 3D models by presenting a relatively low cost and a consequent reproducibility. These models can therefore constitute interesting platforms for the validation of drugs. Bioprinting offers a more controlled way to use hydrogels in the form of bioinks while providing the ability to establish complex 3D architectures that incorporate cellular heterogeneities (27,28). While this method suffers from the same downside as classical hydrogel from which the bioinks are derived from, it remains a method that is improving quickly and has been used to explore cancer related questions (29,30). Self-assembly is another tissue engineering method which is devoid of exogenous biomaterials. It allows the production of the stromal compartment (cell populated ECM scaffold) by the mesenchymal cells themselves. It was especially demonstrated that the use of organ-specific mesenchymal cells to reconstruct the stromal compartment allow the adequate differentiation of the epithelial cells presenting a high degree of histological and functional similarity with the native tissue. Recently, several models of human 3D cancer models have been engineered using the self-assembly method, including basal carcinoma, melanoma, neurofibroma and BCa (31). In fact, it might be the best method to account for the stromal cell heterogeneities, and as we have previously demonstrated, the addition of CAF allows to establish a BCa-like tumor stroma that can triggers partial EMT in the epithelial compartment (32). In addition, all those 3D methods are compatible with omics assays, including scRNA-seq (22), meaning that it would be possible to see if the heterogeneity observed by Wang et al. is maintained in these in vitro models. Overall, a combination of several such models is likely key in testing clinically relevant parameters, such as cell invasion or drug resistance, for targets identified in studies similar to the one presented by Wang et al. in a physiologically relevant setting.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Stem Cell Investigation. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://sci.amegroups.com/article/view/10.21037/sci-2023-004/coif). FB is a tier 2 Canada Research Chair in Tumor Mechanobiology and Cellular Mechanoregulation. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang H, Mei Y, Luo C, et al. Single-Cell Analyses Reveal Mechanisms of Cancer Stem Cell Maintenance and Epithelial-Mesenchymal Transition in Recurrent Bladder Cancer. Clin Cancer Res 2021;27:6265-78. [Crossref] [PubMed]
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- John BA, Said N. Insights from animal models of bladder cancer: recent advances, challenges, and opportunities. Oncotarget 2017;8:57766-81. [Crossref] [PubMed]
- Clark PE, Agarwal N, Biagioli MC, et al. Bladder cancer. J Natl Compr Canc Netw 2013;11:446-75. [Crossref] [PubMed]
- Jiang S, Redelman-Sidi G. BCG in Bladder Cancer Immunotherapy. Cancers (Basel) 2022;14:3073. [Crossref] [PubMed]
- Liu S, Chen X, Lin T. Emerging strategies for the improvement of chemotherapy in bladder cancer: Current knowledge and future perspectives. J Adv Res 2022;39:187-202. [Crossref] [PubMed]
- Lyu T, Lin Y, Wu K, et al. Single-cell sequencing technologies in bladder cancer research: Applications and challenges. Front Genet 2022;13:1027909. [Crossref] [PubMed]
- Chen YP, Zhang Y, Lv JW, et al. Genomic Analysis of Tumor Microenvironment Immune Types across 14 Solid Cancer Types: Immunotherapeutic Implications. Theranostics 2017;7:3585-94. [Crossref] [PubMed]
- Weidle UH, Birzele F. Bladder Cancer-related microRNAs With In Vivo Efficacy in Preclinical Models. Cancer Diagn Progn 2021;1:245-63. [Crossref] [PubMed]
- Orabi H, Bouhout S, Morissette A, et al. Tissue engineering of urinary bladder and urethra: advances from bench to patients. ScientificWorldJournal 2013;2013:154564. [Crossref] [PubMed]
- Dalghi MG, Montalbetti N, Carattino MD, et al. The Urothelium: Life in a Liquid Environment. Physiol Rev 2020;100:1621-705. [Crossref] [PubMed]
- Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 2014;6:114-8. [PubMed]
- van Kessel KE, Zuiverloon TC, Alberts AR, et al. Targeted therapies in bladder cancer: an overview of in vivo research. Nat Rev Urol 2015;12:681-94. [Crossref] [PubMed]
- Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature 2013;501:328-37. [Crossref] [PubMed]
- Biffi G, Tuveson DA. Diversity and Biology of Cancer-Associated Fibroblasts. Physiol Rev 2021;101:147-76. [Crossref] [PubMed]
- Yamamoto Y, Kasashima H, Fukui Y, et al. The heterogeneity of cancer-associated fibroblast subpopulations: Their origins, biomarkers, and roles in the tumor microenvironment. Cancer Sci 2023;114:16-24. [Crossref] [PubMed]
- Öhlund D, Handly-Santana A, Biffi G, et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017;214:579-96. [Crossref] [PubMed]
- Elyada E, Bolisetty M, Laise P, et al. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov 2019;9:1102-23. [Crossref] [PubMed]
- Ohishi T, Koga F, Migita T. Bladder Cancer Stem-Like Cells: Their Origin and Therapeutic Perspectives. Int J Mol Sci 2015;17:43. [Crossref] [PubMed]
- Yan KS, Lin CY, Liao TW, et al. EZH2 in Cancer Progression and Potential Application in Cancer Therapy: A Friend or Foe? Int J Mol Sci 2017;18:1172. [Crossref] [PubMed]
- Hapach LA, Carey SP, Schwager SC, et al. Phenotypic Heterogeneity and Metastasis of Breast Cancer Cells. Cancer Res 2021;81:3649-63. [Crossref] [PubMed]
- Grasset EM, Dunworth M, Sharma G, et al. Triple-negative breast cancer metastasis involves complex epithelial-mesenchymal transition dynamics and requires vimentin. Sci Transl Med 2022;14:eabn7571. [Crossref] [PubMed]
- Brown MS, Abdollahi B, Wilkins OM, et al. Phenotypic heterogeneity driven by plasticity of the intermediate EMT state governs disease progression and metastasis in breast cancer. Sci Adv 2022;8:eabj8002. [Crossref] [PubMed]
- Medle B, Sjödahl G, Eriksson P, et al. Patient-Derived Bladder Cancer Organoid Models in Tumor Biology and Drug Testing: A Systematic Review. Cancers (Basel) 2022;14:2062. [Crossref] [PubMed]
- Saba I, Jakubowska W, Bolduc S, et al. Engineering Tissues without the Use of a Synthetic Scaffold: A Twenty-Year History of the Self-Assembly Method. Biomed Res Int 2018;2018:5684679. [Crossref] [PubMed]
- Galateanu B, Hudita A, Biru EI, et al. Applications of Polymers for Organ-on-Chip Technology in Urology. Polymers (Basel) 2022;14:1668. [Crossref] [PubMed]
- Neufeld L, Yeini E, Pozzi S, et al. 3D bioprinted cancer models: from basic biology to drug development. Nat Rev Cancer 2022;22:679-92. [Crossref] [PubMed]
- Germain N, Dhayer M, Dekiouk S, et al. Current Advances in 3D Bioprinting for Cancer Modeling and Personalized Medicine. Int J Mol Sci 2022;23:3432. [Crossref] [PubMed]
- Kim J, Jang J, Cho DW. Recapitulating the Cancer Microenvironment Using Bioprinting Technology for Precision Medicine. Micromachines (Basel) 2021;12:1122. [Crossref] [PubMed]
- Yi HG. Introduction to bioprinting of in vitro cancer models. Essays Biochem 2021;65:603-10. [Crossref] [PubMed]
- Roy V, Magne B, Vaillancourt-Audet M, et al. Human Organ-Specific 3D Cancer Models Produced by the Stromal Self-Assembly Method of Tissue Engineering for the Study of Solid Tumors. Biomed Res Int 2020;2020:6051210. [Crossref] [PubMed]
- Millet M, Bollmann E, Ringuette Goulet C, et al. Cancer-Associated Fibroblasts in a 3D Engineered Tissue Model Induce Tumor-like Matrix Stiffening and EMT Transition. Cancers (Basel) 2022;14:3810. [Crossref] [PubMed]
Cite this article as: Bordeleau F, Brownell D, Chabaud S, Huot ME, Bolduc S. Recreating heterogeneity of bladder cancer microenvironment to study its recurrences and progression. Stem Cell Investig 2023;10:5.


