Mechanisms determining the fate of hematopoietic stem cells
Hematopoietic stem cells (HSCs) are able to give rise to an organism’s entire blood system. They continuously differentiate into all of the blood cell lineages and possess the capacity for long-term self-renewal. In recent decades, remarkable progress has been achieved in the fight against hematological malignancies. Although novel chemotherapeutic regimens and targeted strategies have been developed, the most powerful weapon is HSC transplantation (HSCT). Since the first clinical trial of HSCT in the early 1960s, millions of patients with malignant or nonmalignant blood diseases have benefitted from HSCT, which is currently the most widely used therapeutic strategy involving stem cells worldwide (1,2). More than 50,000 patients are treated with allogenic or autologous HSCT each year. HSCs have been used to treat patients with leukemia and lymphoproliferative disorders. There are various types of leukemia including acute myeloid leukemia (AML), acute lymphoblastic leukemia, chronic myeloid leukemia (CML), chronic lymphoblastic leukemia, and myelodysplastic syndromes. Lymphoproliferative disorders include Hodgkin lymphomas, non-Hodgkin lymphomas (NHLs), and plasma cell disorders (3). In addition to hematological malignancies, HSCs have been used in the treatment of nonmalignant blood disorders (4), solid tumors (5), autoimmune diseases (6), and immune deficiencies such as human immunodeficiency virus disease (7).
Despite these advances, the application of HSCT is greatly hampered by the lack of sources of HSCs. One solution for this demand is to expand HSCs in vitro. Unfortunately, HSCs are easily differentiated and lose their long-term self-renewal activities in vitro, so the expanded HSCs are unable to reconstitute the recipient’s hematopoietic system (8). Therefore, researchers in the fields of hematology and regenerative medicine have long sought the efficient expansion of functional HSCs. Thus, it is important to study the molecular mechanisms and regulatory networks that modulate the fate of HSCs to gain an understanding of hematopoiesis and to provide critical insight into the clinical applications of HSCs.
The expansion and maintenance of self-renewal in HSCs are regulated by several signaling pathways, such as the Notch (9), Wnt (10), bone morphogenetic protein (BMP) (11), mTOR (12), and Hedgehog (13) pathways, which are in turn regulated by both extrinsic and intrinsic mechanisms (Figure 1) (14). For example, the Wnt signaling required for the self-renewal of HSCs is activated by extracellular proteins (e.g., WNT3A), which then up-regulate some of the genes implicated in self-renewal (e.g., HoxB4 and Notch1) and arrest the HSCs in an undifferentiated stage (10). The stemness of HSCs is niche dependent; it is essential for adult HSCs located in the bone marrow niche (osteoblastic niche and bone marrow vascular niche) (15,16), which include osteoblasts, osteoclasts, perivascular stromal cells, endothelial cells, macrophages, sympathetic neurons, and nonmyelinating Schwann cells (17). It provides physical interaction and secretes many growth factors and chemical modulators, such as NOTCH ligands (JAGGED-1/2) (18,19), WNT proteins (WNT3A) (10,20), BMPs (11), angiopoietin-like factors (21), thrombopoietin (22), stem cell factor (23,24), retinoic acid (25), CXCL12 (26), and E-selectin (27), that activate the regulatory pathways and maintain the self-renewal of HSCs or promote their proliferation. Recently, we found that angiopoietin-like 7 derived from a stromal cell line is capable of promoting the expansion of human HSCs and increasing their repopulation activities via Wnt signaling. This finding provided new insight into the regulation of the fate of HSCs and a new method for in vitro culture of HSCs (28).
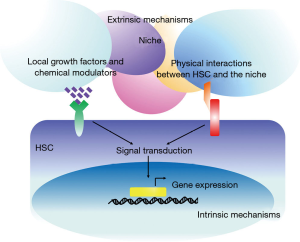
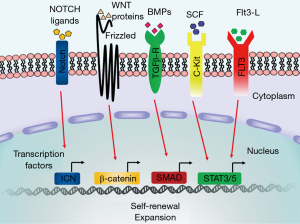
In intrinsic mechanisms, under the cascade of these signaling pathways, transcription factors play the primary role in determining the gene expression profiles of stem cells. The current view is that the fate of HSCs is regulated by competition between transcription factor complexes (29). It is well established that transcription factors such as ICN (18,19), β-catenin (10,20), Myc (30), SMAD (11), STAT3/5 (31), CEBPα (32), HOXB4 (33), GATA2 (34), PU.1 (35), JUNB (36), and GFI1 (37) are necessary for the self-renewal process of HSCs (Figure 2) and that ex vivo over-expression of these code genes may result in expansion of the HSCs by restricting cell differentiation, resetting the cell cycle, and mediating cell division. Self-renewal is activated by diverse signals and regulated by many transcription factors, but these transcription factors are not the sole mediators; for example, Myc, NOTCH, and leukemic fusion proteins together stimulate self-renewal (38,39). Therefore, signaling through multiple pathways is likely to trigger a set of cellular events associated with self-renewal; the transcription factors then make a proper response to these signals and endow a moderate self-renewal process with HSCs. Therefore, self-renewal and expansion occur autonomously in HSCs and are also affected by the niche; the HSCs must remain in a tightly controlled and precisely balanced stage.
Many studies have suggested that leukemia is a stem cell-based disease (40,41). Although the existence and relevance of leukemia-initiating cells (LICs) or leukemia stem cells (LSCs) in acute lymphoblastic leukemia have remained elusive (42,43), LICs have been fairly well described in AML and CML by several research groups (41,44-46). LICs are a subset of cells that have the capacity to self-renew, to give rise to more differentiated progeny, and to maintain the leukemia for long periods. Although LICs and HSCs differ in their production of differentiated cells, they have striking similarities. For example, like HSCs, LICs account for only a small subset of leukemic cells that are capable of extensive proliferation in vitro and in vivo. For most subtypes of AML, the cells capable of transplantation have a (CD34+, CD38–) phenotype, similar to that of HSCs (41,47). In addition, LICs are niche dependent, and xenograft transplantation assays have proven the role of niches in resistance to chemotherapy and in the cell cycle regulation of LICs (48,49). Furthermore, both normal stem cells and LICs depend on SDF-1-mediated CXCR4 signaling for homing and mobilization (50). In addition, many molecular mechanisms that enable self-renewal, such as the Notch (51), Wnt (52,53), angiopoietin (54), and FGF (55) signaling pathways, are common to both normal stem cells and LICs. Increasing evidence suggests that certain subtypes of human leukemia may arise from mutations that accumulate in normal HSCs. For example, in CML, BCR–ABL fusion resulting from t(9;22) was found in HSCs (40). In addition, it has been reported that human CML can be induced in mice by introducing the BCR–ABL fusion protein into normal HSCs (56). Translocation of chromosomes 8 and 21 in HSCs results in RUNX1–ETO fusion and leads to AML (57). Furthermore, preleukemia clones with somatic mutations have been found in the HSCs of patients with AML (58). It has also been reported that the genetic alterations specific for T-cell lymphoma (59), follicular lymphoma (60) and hairy cell leukemia (61) could be traced to the HSC stage. The regulatory network tightly controls and maintains normal HSC function. Disturbances to these systems can lead to dysregulation of the HSCs, impairing their differentiation (62), increasing cell survival (63), and ultimately resulting in the abnormal proliferation of leukemic cells. Therefore, it is reasonable to assume that LICs may be derived from normal HSCs, and further studies should focus on the molecular mechanisms that transform HSCs into LICs.
Some of the biological features of HSCs have now been recognized, but the molecular mechanisms that underlie these properties are still not clearly understood. Investigation of the regulatory mechanisms of HSCs may help us to understand not only the origin of LICs but also to determine a means of expanding functional HSCs in vitro, which would have many beneficial clinical uses.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med 2006;354:1813-26. [PubMed]
- Bordignon C. Stem-cell therapies for blood diseases. Nature 2006;441:1100-2. [PubMed]
- Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA 2010;303:1617-24. [PubMed]
- Prasad VK, Kurtzberg J. Umbilical cord blood transplantation for non-malignant diseases. Bone Marrow Transplant 2009;44:643-51. [PubMed]
- Ljungman P, Urbano-Ispizua A, Cavazzana-Calvo M, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: definitions and current practice in Europe. Bone Marrow Transplant 2006;37:439-49. [PubMed]
- Farge D, Labopin M, Tyndall A, et al. Autologous hematopoietic stem cell transplantation for autoimmune diseases: an observational study on 12 years' experience from the European Group for Blood and Marrow Transplantation Working Party on Autoimmune Diseases. Haematologica 2010;95:284-92. [PubMed]
- Kiem HP, Jerome KR, Deeks SG, et al. Hematopoietic-stem-cell-based gene therapy for HIV disease. Cell Stem Cell 2012;10:137-47. [PubMed]
- Sorrentino BP. Clinical strategies for expansion of haematopoietic stem cells. Nat Rev Immunol 2004;4:878-88. [PubMed]
- Butler JM, Nolan DJ, Vertes EL, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 2010;6:251-64. [PubMed]
- Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 2003;423:409-14. [PubMed]
- Karlsson G, Blank U, Moody JL, et al. Smad4 is critical for self-renewal of hematopoietic stem cells. J Exp Med 2007;204:467-74. [PubMed]
- Iriuchishima H, Takubo K, Matsuoka S, et al. Ex vivo maintenance of hematopoietic stem cells by quiescence induction through Fbxw7α overexpression. Blood. 2011;117:2373-7. [PubMed]
- Bhardwaj G, Murdoch B, Wu D, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol 2001;2:172-80. [PubMed]
- Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature 2008;453:306-13. [PubMed]
- Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003;425:841-6. [PubMed]
- Kiel MJ, Yilmaz OH, Iwashita T, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 2005;121:1109-21. [PubMed]
- Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med 2014;20:833-46. [PubMed]
- Varnum-Finney B, Purton LE, Yu M, et al. The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood 1998;91:4084-91. [PubMed]
- Stier S, Cheng T, Dombkowski D, et al. Notch1 activation increases hematopoietic stem cell self-renewal in vivo and favors lymphoid over myeloid lineage outcome. Blood 2002;99:2369-78. [PubMed]
- Koch U, Wilson A, Cobas M, et al. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood 2008;111:160-4. [PubMed]
- Zhang CC, Kaba M, Ge G, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med 2006;12:240-5. [PubMed]
- Petit-Cocault L, Volle-Challier C, Fleury M, et al. Dual role of Mpl receptor during the establishment of definitive hematopoiesis. Development 2007;134:3031-40. [PubMed]
- Ding L, Saunders TL, Enikolopov G, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012;481:457-62. [PubMed]
- Sasaki T, Mizuochi C, Horio Y, et al. Regulation of hematopoietic cell clusters in the placental niche through SCF/Kit signaling in embryonic mouse. Development 2010;137:3941-52. [PubMed]
- Purton LE, Dworkin S, Olsen GH, et al. RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J Exp Med 2006;203:1283-93. [PubMed]
- Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2m(null) mice. Leukemia 2002;16:1992-2003. [PubMed]
- Winkler IG, Barbier V, Nowlan B, et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med 2012;18:1651-7. [PubMed]
- Xiao Y, Jiang Z, Li Y, et al. ANGPTL7 regulates the expansion and repopulation of human hematopoietic stem and progenitor cells. Haematologica 2015;100:585-94. [PubMed]
- Nerlov C, Graf T. PU. 1 induces myeloid lineage commitment in multipotent hematopoietic progenitors. Genes Dev 1998;12:2403-12. [PubMed]
- Murphy MJ, Wilson A, Trumpp A. More than just proliferation: Myc function in stem cells. Trends Cell Biol 2005;15:128-37. [PubMed]
- L'Hôte CG, Knowles MA. Cell responses to FGFR3 signalling: growth, differentiation and apoptosis. Exp Cell Res 2005;304:417-31. [PubMed]
- Istvanffy R, Kröger M, Eckl C, et al. Stromal pleiotrophin regulates repopulation behavior of hematopoietic stem cells. Blood 2011;118:2712-22. [PubMed]
- Sauvageau G, Thorsteinsdottir U, Eaves CJ, et al. Overexpression of HOXB4 in hematopoietic cells causes the selective expansion of more primitive populations in vitro and in vivo. Genes Dev 1995;9:1753-65. [PubMed]
- Rodrigues NP, Janzen V, Forkert R, et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood 2005;106:477-84. [PubMed]
- Iwasaki H, Somoza C, Shigematsu H, et al. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood 2005;106:1590-600. [PubMed]
- Passegué E, Wagner EF, Weissman IL. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell 2004;119:431-43. [PubMed]
- Zeng H, Yücel R, Kosan C, et al. Transcription factor Gfi1 regulates self-renewal and engraftment of hematopoietic stem cells. EMBO J 2004;23:4116-25. [PubMed]
- Varnum-Finney B, Xu L, Brashem-Stein C, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med 2000;6:1278-81. [PubMed]
- Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 2006;442:818-22. [PubMed]
- Holyoake T, Jiang X, Eaves C, et al. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood 1999;94:2056-64. [PubMed]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730-7. [PubMed]
- Bernt KM, Armstrong SA. Leukemia stem cells and human acute lymphoblastic leukemia. Semin Hematol 2009;46:33-8. [PubMed]
- Rehe K, Wilson K, Bomken S, et al. Acute B lymphoblastic leukaemia-propagating cells are present at high frequency in diverse lymphoblast populations. EMBO Mol Med 2013;5:38-51. [PubMed]
- Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994;367:645-8. [PubMed]
- Graham SM, Jørgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 2002;99:319-25. [PubMed]
- Barnes DJ, Melo JV. Primitive, quiescent and difficult to kill: the role of non-proliferating stem cells in chronic myeloid leukemia. Cell Cycle 2006;5:2862-6. [PubMed]
- Blair A, Hogge DE, Ailles LE, et al. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood 1997;89:3104-12. [PubMed]
- Ishikawa F, Yoshida S, Saito Y, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol 2007;25:1315-21. [PubMed]
- Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood 2009;114:1150-7. [PubMed]
- Tavor S, Petit I, Porozov S, et al. CXCR4 regulates migration and development of human acute myelogenous leukemia stem cells in transplanted NOD/SCID mice. Cancer Res 2004;64:2817-24. [PubMed]
- Ma W, Gutierrez A, Goff DJ, et al. NOTCH1 signaling promotes human T-cell acute lymphoblastic leukemia initiating cell regeneration in supportive niches. PLoS One 2012;7:e39725. [PubMed]
- Wang Y, Krivtsov AV, Sinha AU, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science 2010;327:1650-3. [PubMed]
- Yeung J, Esposito MT, Gandillet A, et al. β-Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell 2010;18:606-18. [PubMed]
- Müller A, Lange K, Gaiser T, et al. Expression of angiopoietin-1 and its receptor TEK in hematopoietic cells from patients with myeloid leukemia. Leuk Res 2002;26:163-8. [PubMed]
- Karajannis MA, Vincent L, Direnzo R, et al. Activation of FGFR1beta signaling pathway promotes survival, migration and resistance to chemotherapy in acute myeloid leukemia cells. Leukemia 2006;20:979-86. [PubMed]
- Wong S, Witte ON. Modeling Philadelphia chromosome positive leukemias. Oncogene 2001;20:5644-59. [PubMed]
- Miyamoto T, Weissman IL, Akashi K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci U S A 2000;97:7521-6. [PubMed]
- Shlush LI, Zandi S, Mitchell A, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 2014;506:328-33. [PubMed]
- Couronné L, Bastard C, Bernard OA. TET2 and DNMT3A mutations in human T-cell lymphoma. N Engl J Med 2012;366:95-6. [PubMed]
- Weigert O, Weinstock DM. The evolving contribution of hematopoietic progenitor cells to lymphomagenesis. Blood 2012;120:2553-61. [PubMed]
- Chung SS, Kim E, Park JH, et al. Hematopoietic stem cell origin of BRAFV600E mutations in hairy cell leukemia. Sci Transl Med 2014;6:238ra71.
- Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol 1976;33:451-8. [PubMed]
- Delia D, Aiello A, Soligo D, et al. bcl-2 proto-oncogene expression in normal and neoplastic human myeloid cells. Blood 1992;79:1291-8. [PubMed]
Cite this article as: Lin S, Zhao R, Xiao Y, Li P. Mechanisms determining the fate of hematopoietic stem cells. Stem Cell Investig 2015;2:10.


