Impact of maternal and neonatal factors on umbilical cord CD34+ cells
Introduction
Stem cells can be defined as undifferentiated cells that have the ability to produce exactly similar cells, capable of dividing indefinitely (self-renewal), and also capable of differentiating into specialized cell lineages (1).
Banking of the umbilical cord blood is the process by which collection and storage of umbilical cord blood is carried out immediately after birth (2). Some of the cord blood banks (CBBs) provide not only cord blood banking, but also banking of umbilical cord tissues, where a small segment of the umbilical cord itself is preserved and stored (3).
The interest in umbilical cord tissues is growing, because they are considered as a very rich source of mesenchymal stem cells, which provide a great potential for the field of regenerative medicine. Many clinical trials are currently investigating the use of Cord-derived haematopoietic as well as mesenchymal stem cells for neurological and autoimmune disorders such as autism, cerebral palsy and type 1 diabetes (4).
To establish a CBB, the number of CD34+ cells in each cord blood unit (CBU) must be sufficient, that’s why maternal and neonatal factors should be considered when selecting CBUs.
Many factors have been suggested to influence the quantity of CD34+ Cell derived from the umbilical cord blood, and that may be responsible for the variations in the reported results. Among these factors; the birth weight, sex of the newborn and maternal age which may have an effect on the concentration of CD34+ cells (5).
The aim of this study was to assess the total number of CD34+ cells in umbilical cord blood and to study the effect of some maternal and neonatal factors such as; maternal age, number of previous live births, presence of any maternal disease, gestational period, mode of delivery (vaginal vs. cesarean delivery), baby’s birth weight, weight of the placental, infant’s gender, and length of umbilical cord and their effect on the CD34+ cells content of CBUs.
Methods
This is an experimental study carried out on the umbilical cord blood sample which was collected in the delivery room in El-Mehalla General Hospital and sent within 24 hours to be processed at the Center of Excellence at Tanta University International Educational Hospital.
All cases were subjected to complete history taking including maternal age, gestation period, and number of previous live births. All new born babies were subjected to full clinical examination.
All samples were collected from the umbilical cord immediately after delivery of the baby and while the placenta is still in utero. The site of cord puncture was sterilized using iodine in a unidirectional movement. A 16-gauge needle connected to a blood donor set containing 35 mL of citrate phosphate dextrose anticoagulant was inserted into the umbilical vein; cord blood was allowed to flow by gravity, and the needle was not removed until the flow of blood had already stopped. UCB units were considered unacceptable if the volume of collected blood was less than 30 mL and/or if the unit delivery for analysis was delayed beyond 24 hours after collection.
Separation of CD 34+cells from cord blood
Separation of mononuclear cells: The cord blood was diluted at a ratio of 2:1 with a clinical buffer, then the diluted cell suspension was carefully layered over high density gradient medium (Ficoll-Paque) in a 15 mL conical tube in a ratio of 2 diluted blood: 1 Ficoll. Samples then centrifuged at 2,000 rpm for 20 minutes at 20 °C in a swinging out bucket rotor without break and the mononuclear cell layer was aspirated carefully undisturbed at the interphase to be transferred to a new 15 mL conical tube. The cell pellet was then washed by adding up to 10 mL of clinical buffer, mixed gently and centrifuged at 1,200 rpm for 15 minutes at 20 °C, then the supernatant was carefully and completely removed (this step was repeated twice) lastly the cell pellet was re-suspended in appropriate amount of clinical buffer (final volume up to 108 total cells/ 300 µL of clinical buffer) (6).
Immune-magnetic purification of CD34 stem cell population
The cell pellet was re-suspended in 1 mL clinical buffer with the CD Microbeads (300 µm) Mixing well for the cell suspension and then refrigerated for 30 minutes, the cells after that were washed with clinical buffer (10 mL) and centrifuged at 1,800 rpm for 20 minutes, then complete aspiration of the supernatant was done followed by suspension of the cells in 500 µL buffer. Column (MS column) was placed in the magnetic field of Mini MACS separator.
The cell suspension was slowly applied into the column after preparation of the column was done by its rinsing using 500 µL clinical buffer. One mL of the clinical buffer was pipetted onto the column. The magnetically labelled cells were immediately flushed out by firmly pushing the plunger into the column. Unlabelled cells that passed through were collected and the column was washed again with the clinical buffer. Washing steps were performed by adding clinical buffer three times (3×500 µL clinical buffer), the new clinical buffer was only added when the column reservoir was completely empty. Total effluent was collected, containing the unlabelled cell fraction. The column was removed from the Mini MACS separator and placed on a suitable collection tube and the purity of the cells was determined by flow cytometry.
Statistical methods
Statistical analysis of the data collected from present study was done using the mean, standard deviation, and Chi-square tests by SPSS V.16. Significance was adopted with P
Results
Samples were collected from 20 deliveries, 19 cesarean section (CS) and one normal vaginal delivery (NVD). The maternal age ranged from 22 to 34 years. They gave birth to 9 males and 11 females.
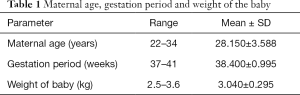
As regard the maternal age: the mean was 28.15 years with a range of 22 to 34 years, the mean for gestational period was 38.4 weeks, ranging from 37 to 41 weeks, the birth weight ranged from 2.5 to 3.6 kg and the mean was 3.040 kg (Table 1).
Full table
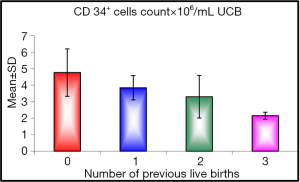
As regards the number of previous live births: five mothers were delivering their first baby (25%), nine were having their second baby (45%), four were giving birth to their third baby (20%) and only two mothers were giving birth to their fourth baby (10%).
CD34+ cells count in the collected samples ranged from 2 to 7×106/mL UCB with a mean of (3.795×106/mL UCB) as shown in (Table 2).

Full table
When we studied the correlation between the number of previous live births we found a significant effect on CD34+ cells count (P value =0.045), with the first baby having higher CD34+ cells count than the second and the second more than the third and the third more than the subsequent births, the range of cells in first baby was (3.1 to 7×106/mL UCB) with mean of (4.760×106/mL UCB), in the second baby the range of cells was 3 to 5.3×106/mL UCB and the mean was (3.844×106/mL UCB), while the third baby had range of cells between (2.5 to 5.2×106/mL UCB) and the mean was (3.300×106/mL UCB) more than subsequent births who had mean of cells (2.150×106/mL UCB) (Figure 1).
Correlation of CD34+ cell count with the sex of the baby was statistically non-significant. The mean CD34+ cell count in males was (3.489×106/mL UCB) compared to (4.045×106/mL UCB) in females (P value =0.326). The route of delivery, maternal age, and gestation period had no significant effect on CD34+ cell count (P value =0.327, 0.186, 0.081 respectively) (Table 3).

Full table
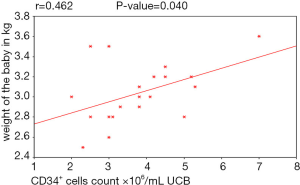
As regards to the weight of the baby there was a positive statistically significant correlation between the birth weight of the baby and CD34+ cells count. An increase of birth weight was associated with to higher CD34+ cells count (P value =0.040) as shown in (Table 3, Figure 2).
Discussion
CD34+ cell concentration in umbilical cord blood is 10-fold higher than in adult peripheral blood. In addition, it was also found that umbilical cord blood progenitors have higher plating efficiency in clonogenic assays (7). Some maternal and fetal factors have been suggested to influence the total number of umbilical cord blood-derived CD34+ cells (5).
The CD34+ cells count in our study ranged from 2 to 7×106/mL UCB with a mean of 3.79±1.22×106/mL UCB. These results were the same of Hussein et al., 2014 (8) who did a study on 124 UCB samples. The mean of CD34+ cell counts was 3.8±1. 9×106/mL UCB however A lower mean CD34+ cell counts (2.2×106/mL UCB) was reported by Al-Deghaither, 2015 (9) in comparison to the mean of CD34+ cells count in our study. This difference in CD34+ cells count between studies can be explained by variation in ethnic groups in addition to maternal, neonatal and obstetric factors related to collecting UCB samples (9).
Birth order in our study included 5 first born babies, 9 second born babies, 4 third born babies and 2 more than third babies. The correlation between birth order and CD34+ cells showed significant effect of birth order on CD34+ cells count. These findings are different from Hussein et al. 2014 (8) who suggested that there was no significant correlation between birth order and UCB volume, total nucleated cell (TNC) count, and CD34+ levels.
Researches done by Jan et al. 2008 (10) and Ballen et al. 2001 (11) agreed with our study that the first baby has a higher CD34+ cell count than subsequent live births. They explained that on the bases that the first pregnancy is usually associated with weakening of the placental vasculature and also they suggested that first babies are usually associated with a longer duration of labor; longer labor has been associated with higher TNC counts, and hence a higher CD34+ cell count which constitute a fraction of the TNC.
But our study was in disagreement with Thame et al. 2004 (12) who stated in their researches that the higher the number of previous live births, the higher the CD34+ cell counts in UCB. They explained their results by that bigger placental size in multiparous mothers thus providing larger volume of cord blood and more CD34+ cells count.
In our study, the sex of the babies from whom the cord blood was collected had no significant effect on CD34+ cells count. Ballen et al., 2001 (11) and Chandra et al., 2012 (13) reported the same results and found no correlation between the baby’s sex and UCB derived CD34+ cells while Nakagawa et al., 2004 (5) showed that UCB from female babies had significantly higher CD34+ cell counts when compared to UCB derived from male babies. Page et al., 2014 (14) and Bassiouny et al., 2015 (15) had shown opposite results to the previous findings. They explained that by stating that male babies have a higher UCB volume, and higher birth weight than females.
In this study the route of delivery had no significant effect on CD34+ cells, which is in agreement with Lim et al. 1994 (16) who did not support any effect that the mode of delivery might have on CD34+ cell counts in UCB. Dimitriou et al., 2006 (17) also assured that whatever the mode of delivery is, it has no effect on either hematopoietic progenitor colony forming unit-granulocyte-macrophage or the UCB content in CD34+ cells.
On the contrary, other investigators observed a higher CD34+ cell concentration and Colony Forming Unit concentration in case of CS than that of normal vaginal mode [Aroviita et al., 2005 (18); Jan et al., 2008 (10) and Chandra et al., 2012 (13)], as an explanation for this finding we can assume that during CS the newborn is held above the placenta before clamping of the cord, causing a gravity-enhanced downward flow of blood into the umbilical cord. Another hypothesis is that CS permits a faster manual extraction of the placenta than in case of NVD, hence reducing the chance of clot formation in the UCB (19).
The maternal age in our study showed no significant correlation when correlated to the CD34+ cells count. This is in agreement with previous reports presented by several studies who stated that there is no significant correlation between maternal age and CD34+ cell count [Nakagawa et al., 2004 (5); Askari et al., 2005 (20); Mancinelli et al., 2006 (21)].
However, Al-Sweedan et al., 2013 (22) reported that maternal age had a positive correlation with CD34+ cell counts. Page et al., 2014 (14) also found that Mothers older than 20 years provide more CBUs CD34+ cell concentration compared to younger mothers
In the present study the range of gestational duration was 37 to 41 weeks and there was non-significant relationship with the CD34+ cell count. Cervera et al. 2006 (23) also in his study demonstrated non-significant association between infant CD34+ cells concentration and neonatal gestational age. Several studies found an inverse statistically significant correlation between number of CD34+ cells and the advancement of gestational age [Surbek et al., 2001 (24); Ballen et al., 2001 (11); Nakagawa et al., 2004 (5). Each extra week of gestation caused a decrease in CD34+ cell concentration by 9% (Ballen et al., 2001 (11)]. The hypothesis that could explain this negative relationship between the gestational age and CD34+ cells is that advancement of gestation is associated with placental aging and the consequently a decline in the blood flow to the fetus which could result in lower counts of CBU CD34+ cells (25).
Lin et al. 2000 (26) also demonstrated that CD34+ cell counts detected in human UCB is significantly higher in very preterm fetuses (from 25 to 29 weeks of gestation) compared to the more mature preterm (from 29 to 35 weeks), and both groups of preterm newborns, showed a higher concentration of CD34+ cells compared to full term babies. On contrast Chandra et al. 2012 (13) reported significant positive correlation between the gestational age CD34+ cell concentration in UCB because CD34+ cell concentration may vary significantly with the process of hematopoiesis during deferent phases of gestation, an explanation for this finding is that with advancement of gestational age and placental aging, the fetus would become progressively hypoxic, triggering the defense mechanisms that increases the number of hematopoietic cells in the UCB (25).
Birth weight was another parameter statistically analyzed in our study and showed that there was a statistically significant positive correlation between the weight of the baby at birth and CD34+ cells count. Several studies by Nakagawa et al., 2004 (5); Mancinelli et al., 2006 (21); Chandra et al., 2012 (13); stated that the increase in birth weight was associated with higher CD34+ cell numbers. Ballen et al. 2001 (11) reported that each 500 g increase in birth weight contributed to an increase of about 11% in the TNC count, as well as a 28% increase in CD34+ cell. Page et al., 2014 (14) showed that babies who weigh more than 3,500 g had higher CD34+ cell counts.
Limitations of the study
One of the main limitation of this study is the low number of the studied cases.
Conclusions
Umbilical cord blood-derived CD34+ cell count can be influenced by both maternal and neonatal factors such as the number of previous live births and weight of the baby had a significant effect on umbilical cord blood-derived CD34+ cells while sex of the baby, delivery route, maternal age and gestation period had no significant effect on CD34+ cells.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by REC (Research Ethical Committee) of the Faculty of Medicine, Tanta University (Protocol ID: 3164/08/17). Written informed consent was obtained from the mothers participating in the study before cord blood sampling, with approval of cord blood analysis as well as data usage and publication.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kørbling M, Estrov A. Adult stem cells for tissue repair: a new therapeutic concept? N Engl J Med 2003;349:570-82. [Crossref] [PubMed]
- Cooper CA, Severson MR. Cord blood and tissue banking: supporting expectant parent’s decision making. Int J Child Educ 2013;28:62-8.
- Fannin M. Personal stem cell banking and the problem with property. Soc Cult Geogr 2011;12:339-56. [Crossref]
- El Omar R, Beroud J, Stolz J, et al. Umbilical cord mesenchymal stem cells: The new gold standard for mesenchymal stem cell-based therapies? Tissue Eng Part B Rev 2014;20:523-44. [Crossref] [PubMed]
- Nakagawa R, Watanabe T, Kawano Y, et al. Analysis of maternal and neonatal factors that influence the nucleated and CD34+Cell yield for cord blood banking. Transfusion 2004;44:262-7. [Crossref] [PubMed]
- Aktas M, Radke TF, Strauer BE, et al. Separation of adult bone marrow mononuclear cells using the automated closed separation system Sepax. Cytotherapy 2008;10:203-11. [Crossref] [PubMed]
- Wyrsch A, Carbonare V, Jansen W, et al. Umbilical cord blood from preterm human fetuses is rich in committed and primitive hematopoietic progenitors with high proliferative and self-renewal capacity. Exp Hematol 1999;27:1338-45. [Crossref] [PubMed]
- Hussein AA, Bawadi RM, Tahtamouni LH, et al. Feasibility of collecting umbilical cord blood in Jordan and the effect of maternal and neonatal factors on hematopoietic stem cell content. Mediterr J Hematol Infect Dis 2014;6:e2014019. [Crossref] [PubMed]
- Al-Deghaither SY. Impact of maternal and neonatal factors on parameters of hematopoietic potential in umbilical cord blood. Saudi Med J 2015;36:704-12. [Crossref] [PubMed]
- Jan RH, Wen SH, Shyr MH, et al. Impact of maternal and neonatal factors on CD34+ cell count, total nucleated cells, and volume of cord blood. Pediatr Transplant 2008;12:868-73. [Crossref] [PubMed]
- Ballen KK, Wilson M, Wuu J, et al. Bigger is better: maternal and neonatal predictors of hematopoietic potential of umbilical cord blood units. Bone Marrow Transplant 2001;27:7-14. [Crossref] [PubMed]
- Thame M, Osmond C, Bennett F, et al. Fetal growth is directly related to maternal anthropometry and placental volume. European journal of clinical nutrition 2004;58:894. [Crossref] [PubMed]
- Chandra T, Afreen S, Kumar A, et al. Does umbilical cord blood-derived CD34+ cell concentration depend on the weight and sex of a full-term infant?. J Pediatr Hematol Oncol 2012;34:184-7. [Crossref] [PubMed]
- Page KM, Mendizabal A, Betz-Stablein B, et al. Optimizing donor selection for public cord blood banking: influence of maternal, infant, and collection characteristics on cord blood unit quality. Transfusion 2014;54:340-52. [PubMed]
- Bassiouny MR, El-Chennawi F, Mansour AK, et al. Optimal method for collection of umbilical cord blood: an Egyptian trial for a public cord blood bank. Transfusion. 2015;55:1263-8. [Crossref] [PubMed]
- Lim FTH, Winsen LV, Willemze R, et al. Influence of delivery on numbers of leukocytes and leukocyte subpopulations, and hematopoietic progenitor cells in human umbilical cord blood. Blood Cells 1994;20:547-58; discussion 558-9. [PubMed]
- Dimitriou H, Perdikogianni C, Stiakaki E, et al. The impact of mode of delivery and gestational age on cord blood hematopoietic stem/progenitor cells. Ann Hematol 2006;85:381-5. [Crossref] [PubMed]
- Aroviita P, Teramo K, Hiilesmaa V, et al. Cord blood hematopoietic progenitor cell concentration and infant sex. Transfusion 2005;45:613-21. [Crossref] [PubMed]
- Nunes RD, Zandavalli FM. Association between maternal and fetal factors and quality of cord blood as a source of stem cells. Rev Bras Hematol Hemoter 2015;37:38-42. [Crossref] [PubMed]
- Askari S, Miller J, Chrysler G, et al. Impact of donor-and collection-related variables on product quality in ex utero cord blood banking. Transfusion 2005;45:189-94. [Crossref] [PubMed]
- Mancinelli F, Tamburini A, Spagnoli A, et al. Optimizing umbilical cord blood collection: impact of obstetric factors versus quality of cord blood units. Transplant Proc 2006;38:1174-6. [Crossref] [PubMed]
- Al-Sweedan SA, Musalam L, Obeidat B. Factors predicting the hematopoietic stem cells content of the umbilical cord blood. Transfus Apher Sci 2013;48:247-52. [Crossref] [PubMed]
- Cervera A, Lilli R, Garcia-Sanchez F, et al. Flow Cytometric Assessment of Hematopoietic Cell Subsets in Cryopreserved Preterm and Term Cord Blood, Influence of Obstetrical Parameters, and Availability for Transplantation. American Journal of Hematology 2006;81:397-410. [Crossref] [PubMed]
- Surbek DV, Danzer E, Steinmann C, et al. Effect of preeclampsia on umbilical cord blood hematopoietic progenitor-stem cells. Am J Obstet Gynecol 2001;185:725-9. [Crossref] [PubMed]
- Mousavi SH, Abroun S, Zarrabi M, et al. The effect of maternal and infant factors on cord blood yield. Pediatr Blood Cancer 2017;64:e26381. [Crossref] [PubMed]
- Lin Y, Weisdorf DJ, Solovey A, et al. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest 2000;105:71-7. [Crossref] [PubMed]
Cite this article as: Rowisha MA, El-Shanshory MR, El-Hawary EE, Ahmed AY, Altoraky SR. Impact of maternal and neonatal factors on umbilical cord CD34+ cells. Stem Cell Investig 2020;7:5.