
Isolation of umbilical cord mesenchymal stem cells using human blood derivatives accompanied with explant method
Introduction
Mesenchymal stem cells (MSCs) have been increasingly used in clinical trials in recent years because of their ability to repair damaged tissues and healing wound beside their immunomodulatory properties. MSCs are characterized based on their ability of adherence to the plastic surface, trilineage differentiation ability—adipocytes, osteocytes, and chondrocytes, and expressing MSCs specific surface markers CD90, CD73, and CD105 in addition to lack of expression of CD34, CD45, and CD14 (1,2). Many sources have been used to obtain MSCs such as bone marrow, adipose tissue, dental pulp and umbilical cord blood and tissues. Among these sources, umbilical cord tissue is the most attractive source of MSCs since isolation procedure is easier and non-invasive compared to the other sources. In fact, the proliferation of umbilical cord tissue-derived MSCs (UCMSCs) are greater than other sources (3).
Different protocols are used to isolate MSCs which differ from each other depending on the source of cells and the utility of the isolated cells. In this context, several laboratories are optimizing their own protocols (4). Various studies have compared protocols for isolating MSCs, either from different sources or by using different supplements (5,6). In this regard, there are mainly two types of methods to isolate MSCs from umbilical cord tissues, enzymatic methods which depend on digestion of umbilical cord tissues using collagenase or collagenase and hyaluronidase solutions, and explant methods which depend on migration ability of MSCs from tissue explants without using digestive enzymes. Although enzymatic methods are less time consuming, the explant method is simpler and cost-effective. In fact, using digestive enzyme raise the risk of degradation of the cellular external lamina and prevent MSCs from adhering to the surface of culture vessels (7,8).
On the other hand, fetal bovine serum (FBS) is considered to be the gold standard for cell culture media as it provides cells with many growth and attachment factors. However, FBS has many limitations when using isolated and expanded cells in clinical fields such as inducing immunological reactions due to its animal source and contamination risk by bacteria or viruses (9). In order to overcome FBS disadvantages, many supplements have been tested for isolation and expansion of MSCs such as human blood derivatives like human blood plasma, platelet lysate (PL) (10), and cord blood serum (CBS) (11). The human blood derivatives have been used as FBS alternatives and found to be suitable for both isolation and expansion of MSCs. CBS is prepared from cord blood and contain many growth factors (12), while, PL is prepared from platelet enriched plasma by several methods such as sonication and chemical or physiological stimulation and freezing-thawing which induces the release of growth factors from platelets that are required for both proliferation and growth of MSCs. PL has been proved to support growth, proliferation, and differentiation of stem cells (13-15).
Although there is a difference between plasma and serum with reference to their components, the comparison between PL and CBS as FBS alternatives is not fully addressed for isolation of MSCs from umbilical cord tissues. Moreover, recent comparative studies are focused on the expansion of MSCs more than isolation (6,12,16,17). So, the aim of this study was to compare between PL and CBS as FBS alternatives in isolation of umbilical cord-derived MSCs (UCMSCs) using explant method. To undertake this, we cultured umbilical cord tissue under different conditions. The isolated cells were counted and characterized based on their immunophenotype and differentiation ability.
Methods
Preparation of pooled CBS
Cord blood was collected in 50 mL falcon tubes without anticoagulant from healthy pregnant women after delivery. Prior to the collection, approval from Damascus University ethical committee and written informed consents from all participants were taken. The blood was left for 2–4 hours and then centrifuged at 1,000 g for 30 min at 20 °C. The sera were collected, pooled and filtered using 0.22 µm filters (Ministart, Sartorius Stedim Biotech, Germany). Finally, pooled serum was aliquoted and stored at −20 °C until further use.
Preparation of pooled PL
PL was prepared from platelet rich plasma (PRP) with a platelet count of ≥5×106 cells/mL, obtained from Damascus university blood transfusion center. Three bags of PRP were pooled, frozen at −80 °C and thawed at 37 °C. The freeze/thaw cycle was repeated three times. Thereafter, PL was centrifuged at 15,000 g for 20 minutes at 4°C, and filtrated through 0.22-µm filters (Ministart, Sartorius Stedim Biotech, Germany). Heparin (2 U/mL) (Syrbio, Syria) was added to filtered PL and the aliquots were stored at −20 °C until further use.
Isolation of MSC from umbilical cord tissues
Three umbilical cords were collected from healthy pregnant women during cesarean deliveries in Obstetrics & Gynecology University Hospital, Damascus after taken approval from Damascus University ethical committee and written informed consents from all participants. Isolation of MSCs was established as previously described (18). The samples were collected in phosphate-buffered saline (PBS) (Euroclone, Italy), and transferred to the laboratory where they were washed with PBS and cut into 5 cm2 segments. The segments were cut longitudinally and blood vessels were removed. The segments were transferred to 25 cm2 flasks and cultured in DMEM supplemented with antibiotics, 100 U/mL penicillin and 100 µg/mL streptomycin (Euroclone, Italy). Each sample was cultured in three media which were supplemented with either 10% PL or 10% CBS or 10% FBS. Flasks incubated at 37 °C in a humidified atmosphere with 5% CO2 and were left undisturbed for 7 days. After the initial week-long incubation period, the medium was changed for the first time and thereafter, it was changed every 3–4 days. After 2 weeks, the UC explants were removed and the adherent cells were allowed to expand until day 21 where the cells were detached using 0.05% trypsin-EDTA (Euroclone, Italy). The cells were then quantified using the trypan blue method and were subcultured. Morphology of MSCs was assessed using inverted microscopeIX50 (Olympus, Japan).
Expansion of MSCs and crystal violet staining
Cells at passage 3 were cultured at density 1,000 cells/well in a 12 well plate containing DMEM medium supplemented with either 10% PL or 10% CBS or 10% FBS. The media were changed twice a week. After two weeks, cells were stained with 0.1% crystal violet solution (Carl Roth, Germany).
In vitro differentiation
Cells at passage 3 were cultured at density 5,000 cells/well in 96 well-plates containing DMEM medium supplemented with either 10% PL or 10% CBS or 10% FBS. When the cells reached 70–80% confluence, they were induced to differentiate into adipocytes using adipogenic differentiation medium (hMSC adipogenic differentiation kit, Euroclone, Italy) or into osteocytes using osteogenic differentiation medium (hMSC osteogenic differentiation kit, Euroclone, Italy). After three weeks of induction, the cells were stained by 1% sudan III for 5 min for adipogenic differentiation and by 2% alizarin red (pH 4.2) for 20 min for osteogenic differentiation. Images were taken by inverted microscope IX50 (Olympus, Japan).
Immunophenotypic characterization
The cells in passage 3, which were isolated and expanded in medium supplemented with either 10% PL or 10% CBS or 10% FBS, were washed with PBS and incubated with CD90-FITC (Santa Cruz Biotechnology, USA), CD44-PE (BioRad, USA), CD105-PE (Invitrogen, USA), CD14-FITC (BioRad, USA) CD34-PE (BD Bioscience, USA) and CD45-FITC (Invitrogen, USA). The FACSCalibur (Becton Dickinson, USA) was used to analyze the samples and resultant data was analyzed with CellQuest software (Becton Dickinson, USA) and Flowing Software V2.5.1 (Turku Centre for Biotechnology, Finland).
Statistical analysis
Data was analyzed using SPSS V16.0 and presented in the format mean ± standard error. To calculate p-values, we used Student's T test and considered significant difference between groups when P<0.05.
Results
Isolation of MSCs from umbilical cord tissue
Adherent cells appeared at edges of umbilical cord explants after 10–12 days of culture. Migrated cells from explants cultured in medium supplemented with either 10% PL or 10% CBS or 10% FBS covered a wide area of flasks after 21 days of culture.
Isolated cells from explants showed a typical morphology of MSCs, defined by fibroblast-like morphology with multiple nucleoli and no apparent difference in morphology was observed between isolated cells using medium supplemented with either PL or CBS or FBS (Figure 1A,B,C,D,E,F). The number of isolated cells using human blood derived supplements CBS or PL were higher than those isolated using FBS. The number of isolated cells using PL was 2-fold higher than FBS, while the number of those in CBS was about 10-fold higher as compared with FBS (Figure 1G).
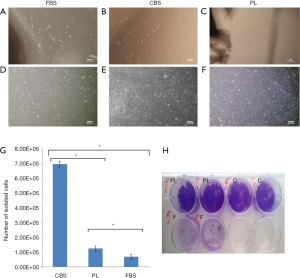
Expansion of MSCs and crystal violet staining
The intensity of the crystal violet staining of expanded cells in passage 3 indicated that using human blood-derived supplements (10% CBS or 10% PL) promote proliferation of MSCs more than 10% FBS (Figure 1H).
In vitro differentiation
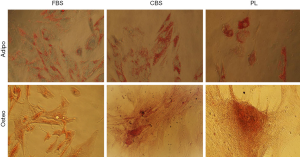
Differentiation of MSCs expanded in either 10% PL or 10% CBS or 10% FBS media at passage 3 demonstrated retention of differentiation potential into osteocytes and adipocytes. MSCs cultured in medium supplemented with either CBS or PL or FBS and differentiated towards osteocytes showed positive staining for alizarin red, indicating the presence of calcium deposits for cultured cells, while the cells which differentiated towards adipocytes were positive for Sudan III indicating accumulation of lipid vacuoles (Figure 2).
Immunophenotyping
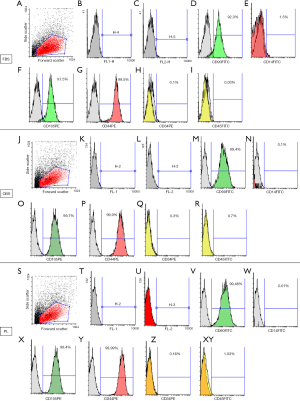
Isolated and expanded cells in medium supplemented with either CBS or PL or FBS were positive for MSCs markers (CD90, CD105, and CD44) and negative for surface markers (CD34, CD45) confirmed by flow cytometry analysis. Nonetheless, expression of MSCs markers (CD90, CD105, and CD44) was considerably higher for cells isolated and expanded in medium supplemented with either CBS or PL than those in FBS as shown in (Figure 3).
Discussion
Isolating sufficient number of MSCs is an essential step for using these cells in clinical and research applications (3). Human blood-derived supplements—CBS and PL—are proven to be a safe and effective xeno-genic alternative of FBS in isolation and expansion of MSCs (6,11,12). Previous studies have focused on the effect of Human-derived supplements on isolation or expansion of MSCs derived from different sources such as adipose tissue (19), bone marrow, and umbilical cord (5,20). Although recent reports showed that platelet lysate or CBS could be used with explant method to isolate MSCs, the comparison between CBS and PL has not be reported and our study is the first to compare between cord blood serum and platelets lysates with explant method in isolation of MSCs from umbilical cord tissue (10,21,22).
In this study, we compared the effect of CBS and PL-supplemented media on isolation of UCMSCs using explant method as a simple protocol with minimal manipulation of umbilical cords. Our results showed that both CBS and PL maintained stemness characteristics of MSCs when used in isolation or expansion, indicated by their fibroblastic morphology, differentiation ability, and expression of MSCs surface markers. In fact, isolated cells using CBS and PL-supplemented media showed similar fibroblastic morphology (Figure 1A,B,C,D,E,F) and adipogenic and osteogenic differentiation ability (Figure 2) compared to FBS supplemented medium. Notably, expression of MSCS surface markers (CD90, CD105 and CD44) was considerably higher for cells isolated and expanded in medium supplemented with either CBS or PL than those in FBS (Figure 3).
Here we showed that human blood derivatives supplements—CBS and PL—are better than FBS when used as a medium supplement in isolation of UCMSCs. Moreover, the number of isolated cells by CBS supplemented media was higher than FBS by about 10-fold, while the number of cells isolated using PL supplemented media was higher than FBS by about 2-fold (Figure 1G). Thus, CBS was the most effective supplement to isolate MSCs comparing to PL and FBS. These results can be explained by the fact that CBS and PL contain many human derived growth factors which promote immigration and proliferation of MSCs better than FBS which contains animal derived growth factors. Moreover, the difference in the content of plasma (PL) and serum (CBS) may affect immigration and proliferation of MSCs. In addition, both CBS and PL contains elevated concentration of many growth factors which play important effects on cells proliferation and differentiation (13,22). We also noticed that use of CBS and PL supplemented media in expanding MSCs is more effective than FBS which was indicated by outgrowths of MSCs that formed monolayer of cells on surface of culture wells when cultured in PL or CBS supplemented media in contrast of FBS condition that formed small separated colonies as showed by crystal violet staining (Figure 1H).
Our results were similar to Kandoi S et al., who used a PL and Khushnuma et al., who used a CBS with bFGF to isolate UCMSCs. However, those studies did not compare both PL and CBS with each other (11,23). In this context, our data suggest that using CBS in isolation of UCMSCs along with explant method is an effective xeno-free method and provides a higher yield of isolated cells compared to both FBS and PL. However, both PL and CBS are more effective than FBS for expanding MSCs.
The importance of our results relates to the potential clinical applications of CBS and PL supplemented media with explant method in isolating UCMSCs. Indeed, CBS and PL are xeno-free supplements which eliminates undesirable effect of FBS such as the risk of transmitting pathogens and trigger xenogeneic immune responses in clinical applications. In addition, isolating a sufficient number of MSCs will reduce long term extended cultures for expansion of MSCs. As a matter of fact, the study findings could be relevant for clinical application since PL could be prepared from Patient’s own blood.
Conclusions
Our results suggest that although all supplements maintain stemness characteristics of MSCs when used to isolate those cells by explant method, using human blood derived supplements in isolating MSCs from umbilical cord tissue is more effective than FBS supplements. CBS is the most effective supplement as compared to FBS and PL.
Finally, use of CBS and PL supplemented media in the expansion of MSCs shall promote better proliferation than FBS supplemented media. Further investigation should focus on the efficiency and potential advantages of CBS and PL in explant isolation media compared with other enzymatic method and assessing the effects of using PL with MSCs in the clinical setup.
Acknowledgments
The main body of this work was carried out at the Department of Pharmaceutical Biotechnology at the National Commission for Biotechnology, Damascus, Syria. The authors thank Dr. Alia Alassad, for her kind help.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Prior to the collection, approval from Damascus University ethical committee (No. 57.2016.1.10) and written informed consents from all participants were taken.
References
- Wei X, Yang X, Han ZP, et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacol Sin 2013;34:747-54. [Crossref] [PubMed]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. [Crossref] [PubMed]
- Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep 2015. [Crossref] [PubMed]
- Iftimia-Mander A, Hourd P, Dainty R, et al. Mesenchymal stem cell isolation from human umbilical cord tissue: understanding and minimizing variability in cell yield for process optimization. Biopreserv Biobank 2013;11:291-8. [Crossref] [PubMed]
- Ben Azouna N, Jenhani F, Regaya Z, et al. Phenotypical and functional characteristics of mesenchymal stem cells from bone marrow: comparison of culture using different media supplemented with human platelet lysate or fetal bovine serum. Stem Cell Res Ther 2012;3:6. [Crossref] [PubMed]
- Pham PV, Vu NB, Pham VM, et al. Good manufacturing practice-compliant isolation and culture of human umbilical cord blood-derived mesenchymal stem cells. J Transl Med 2014;12:56. [Crossref] [PubMed]
- Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells 2007;25:2886-95. [Crossref] [PubMed]
- Yoon JH, Roh EY, Shin S, et al. Comparison of explant-derived and enzymatic digestion-derived MSCs and the growth factors from Wharton's jelly. Biomed Res Int 2013;2013:428726.
- Fernandez-Rebollo E, Mentrup B, Ebert R, et al. Human Platelet Lysate versus Fetal Calf Serum: These Supplements Do Not Select for Different Mesenchymal Stromal Cells. Sci Rep 2017;7:5132. [Crossref] [PubMed]
- Astori G, Amati E, Bambi F, et al. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future. Stem Cell Res Ther 2016;7:93. [Crossref] [PubMed]
- Shetty P, Bharucha K, Tanavde V. Human umbilical cord blood serum can replace fetal bovine serum in the culture of mesenchymal stem cells. Cell Biol Int 2007;31:293-8. [Crossref] [PubMed]
- Kinzebach S, Bieback K. Expansion of Mesenchymal Stem/Stromal cells under xenogenic-free culture conditions. Adv Biochem Eng Biotechnol 2013;129:33-57. [Crossref] [PubMed]
- Bieback K. Platelet lysate as replacement for fetal bovine serum in mesenchymal stromal cell cultures. Transfus Med Hemother 2013;40:326-35. [Crossref] [PubMed]
- Fekete N, Gadelorge M, Furst D, et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: production process, content and identification of active components. Cytotherapy 2012;14:540-54. [Crossref] [PubMed]
- Hemeda H, Giebel B, Wagner W. Evaluation of human platelet lysate versus fetal bovine serum for culture of mesenchymal stromal cells. Cytotherapy 2014;16:170-80. [Crossref] [PubMed]
- Kocaoemer A, Kern S, Kluter H, et al. Human AB serum and thrombin-activated platelet-rich plasma are suitable alternatives to fetal calf serum for the expansion of mesenchymal stem cells from adipose tissue. Stem Cells 2007;25:1270-8. [Crossref] [PubMed]
- Lykov AP, Bondarenko NA, Surovtseva MA, et al. Comparative Effects of Platelet-Rich Plasma, Platelet Lysate, and Fetal Calf Serum on Mesenchymal Stem Cells. Bull Exp Biol Med 2017;163:757-60. [Crossref] [PubMed]
- Hassan G, Kasem I, Soukkarieh C, et al. A Simple Method to Isolate and Expand Human Umbilical Cord Derived Mesenchymal Stem Cells: Using Explant Method and Umbilical Cord Blood Serum. Int J Stem Cells 2017;10:184-92. [Crossref] [PubMed]
- Riis S, Nielsen FM, Pennisi CP, et al. Comparative Analysis of Media and Supplements on Initiation and Expansion of Adipose-Derived Stem Cells. Stem Cells Transl Med 2016;5:314-24. [Crossref] [PubMed]
- Dos Santos VT, Mizukami A, Orellana MD, et al. Characterization of Human AB Serum for Mesenchymal Stromal Cell Expansion. Transfus Med Hemother 2017;44:11-21. [Crossref] [PubMed]
- Wang L, Yang Y, Zhu Y, et al. Characterization of placenta-derived mesenchymal stem cells cultured in autologous human cord blood serum. Mol Med Rep 2012;6:760-6. [Crossref] [PubMed]
- Mirabet V, Solves P, Minana MD, et al. Human platelet lysate enhances the proliferative activity of cultured human fibroblast-like cells from different tissues. Cell Tissue Bank 2008;9:1-10. [Crossref] [PubMed]
- Kandoi S. Evaluation of platelet lysate as a substitute for FBS in explant and enzymatic isolation methods of human umbilical cord MSCs. Sci Rep 2018;8:12439. [Crossref] [PubMed]
Cite this article as: Hassan G, Kasem I, Antaki R, Mohammad MB, AlKadry R, Aljamali M. Isolation of umbilical cord mesenchymal stem cells using human blood derivatives accompanied with explant method. Stem Cell Investig 2019;6:28.


