Philadelphia-positive lymphoblastic lymphoma: a case report and review of the literature
Lymphoblastic lymphoma (LBL) is a rare disease accounting for approximately 8% of all lymphoid malignancies. Bone marrow involvement <25% is the formal cut-off which distinguishes LBL from acute lymphoblastic leukemia (ALL) but the World Health Organization encodes both LBL and ALL as one disease. In ALL there are well-defined parameters, i.e., cytogenetic aberrations, that lead the clinician to correctly stratify patients’ risk: these are not well studied in LBL.
We report here a case of a 31-year-old male with unprecedented medical history whose first access to the hospital was in August 2015 complaining about constant back pain not responsive to pain meds, night sweats and fever.
Biochemistry work-up showed mild normocytic anemia, normal WBC and platelets counts as well as LDH and B2 microglobulin and negative HIV, HBV and HCV serology.
A total body CT scan was performed and showed a paravertebral mass (64×80 mm) between 4° and 6° rib with osteolytic aspect and involvement of D4-D5 intervertebral foramen. CT was followed by PET-scan which confirmed the presence of the mass with high metabolic activity in the aforementioned locations.
A biopsy of the mass was done and came back diagnostic of B-LBL. Contemporarily the bone marrow was analyzed: the cytofluorimetric analysis didn’t detect any B-lymphoblasts but RT-PCR showed low molecular level BCR-ABL p190 positivity (ratio, 0.4718%). At this point we extracted RNA from the mass sample, and we performed RQ-PCR for BCR-ABL/ABL with a confirmed positive level of 69.76%. BCR-ABL positivity was also confirmed on peripheral blood with a BCR-ABL/ABL ratio of 0.41%.
Notably, the FISH analysis on bone marrow for Philadelphia positive cells was negative, whereas FISH analysis on the biopsy specimen was positive for BCR/ABL fusion in 81% of the cells.
The patient initiated treatment with steroids 7 days prior to starting a daily dose of imatinib 800 mg which was rapidly switched to dasatinib 140 mg/day. At the same time a diagnostic-therapeutic lumbar puncture was performed with the injection of intrathecal Methotrexate (MTX) and cytarabine: the cerebrospinal fluid (CSF) analysis came back clear. Steroids therapy was continued for 3 weeks after the introduction of tyrosin-kinase inhibitor (TKI) and then rapidly tapered until complete suspension in 10 days.
After 1 month of TKIs, first imatinib then dasatinib, a CT scan with contrast was repeated showing consistent reduction of the mass size. One course of zoledronic acid was administered due to the osteolytic aspects of the mass.
Other three lumbar puncture were performed beyond the one at diagnosis: they were all negative for disease infiltration and prophylactic cytarabine, methotrexate and dexamethasone were injected in the CSF.
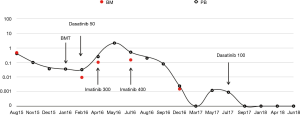
Successive molecular evaluations on peripheral blood and bone marrow were conducted monthly with a slow clearance of BCR-ABL (see Figure 1).
In December 2015, just before transplantation, patient was treated with involved field radiotherapy (12 Gy total) on the residual paravertebral lesion.
The patient then underwent allogeneic bone marrow transplant from matched sibling donor in January 2016 using the standard ALL Cyclophosphamide-TBI conditioning regimen. PET scan made just before transplantation showed complete disappearance of cancer, although a low level of BCR-ABL positivity was still slightly detectable. The procedure was well tolerated; GVHD prophylaxis included methotrexate, ATG and CSA. After transplant the patient suffered from grade II gut GVHD which was managed with high dose steroids at first and then tapered until complete discontinuation.
According to the centre policy and the pre-transplant BCR/ABL positivity, on day +45 after transplant the patient was started on dasatinib 50 mg/day, then shifted after 1 month to imatinib 300 mg/day due to poor hematological tolerance. Imatinib was increased at the standard dose of 400 mg/day in July 2016 as soon as the hematological tolerance improved.
After transplant evaluation of BCR/ABL and chimerism were performed monthly and the persistent molecular positivity led to shift therapy from imatinib to dasatinib in August 2017. Since dasatinib introduction, which was well tolerated with no side effects at all, BCR-ABL/ABL level dropped to 0% and stayed molecular negative in every reverse transcription polymerase chain reaction (RT-PCR) evaluation.
Thirty months have passed since transplantation and the patient continues hematological follow-up, he’s back to work without complaining about any side effects neither from transplant nor dasatinib maintenance therapy.
Discussion
Philadelphia positive LBL is a rare entity which represents a real challenge for hematologists: there’s the chance that this entity is often underdiagnosed especially in departments where qualitative PCR for BCR/ABL rearrangement is not routinely done; once doctors are able to catch the diagnosis the role of usual ALL negative risk factors such as hypodiploidy and/or other abnormal karyotype are not well defined (1) and that can lead to a real dilemma about induction therapy and consolidation.
Literature about Ph positive lymphoblastic lymphoma is very scarce: there’s a case-report about a 27 years old man with primary testicular Ph-positive B LBL, for which fluorescent in-situ hybridization (FISH) for the Philadelphia chromosome was performed only at relapse when the patient already had suffered from complications of intensive chemo regimen and showed central nervous system (CNS) progression which led him to death (2). The case presented here is very similar to another patient with lymphoblastic lymphoma diagnosed on a 77 years old man whose story started with back pain leading to the detection of a Ph positive lymphoblastic mass situated on L5 with a secondary localization on the testicles: the man was treated with hyper-CVAD backbone plus dasatinib, then imatinib and finally nilotinib; he relapsed and died just before starting ponatinib plus blinatumomab combination as salvage therapy 4 years after the diagnosis (3).
Treatment of LBL, either B- or T-cell is based today on ALL protocols which led to an improvement of long-term outcome (4). No recommendation are made for Ph positive lymphoblastic lymphoma because of lack of experience on a large scale; to our knowledge this is the first case report about a Philadelphia positive LBL patient who is not only alive but also in a good clinical status and complete remission 3 years from diagnosis using a chemo-free regimen in induction (as in the Italian GIMEMA protocols fashion about Ph positive ALL), allo-transplant as consolidation from sibling donor and 2GTKI maintenance. This case-report has also the aim to underline the importance of testing for BCR-ABL rearrangement all the masses that are compatible with B-cell LBL as a positivity could dramatically change the treatment of the patient.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Alshomar A, El Fakih R. Philadelphia chromosome-positive lymphoblastic lymphoma-Is it rare or underdiagnosed? Hematol Oncol Stem Cell Ther 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Zhu J, Zhang S, Zhu L, et al. Primary testicular Ph-positive B lymphoblastic lymphoma: an unusual presentation and review. Cancer Biol Ther 2015;16:1122-7. [Crossref] [PubMed]
- Boddu P, Yin CC, Kanagal-Shamanna R, et al. An Unsuspected Finding of t(9;22): A Rare Case of Philadelphia Chromosome-Positive B-Lymphoblastic Lymphoma. Case Rep Hematol 2017;2017:2413587. [Crossref] [PubMed]
- Cortelazzo S, Ferreri A, Hoelzer D, Ponzoni M. Lymphoblastic lymphoma. Crit Rev Oncol Hematol 2017;113:304-17. [Crossref] [PubMed]
Cite this article as: Dragani M, Andreani G, Fava C, Daraio F, Gottardi E, Giugliano E, Nicoli P, Rege-Cambrin G. Philadelphia-positive lymphoblastic lymphoma: a case report and review of the literature. Stem Cell Investig 2019;6:17.