Acute myeloid leukemia remodels endosteal vascular niche into a leukemic niche
At steady state, normal hematopoiesis occurs in highly specialized bone marrow (BM) niches that regulate self-renewal, quiescence and differentiation of hematopoietic stem cells (HSCs) to maintain continuous supply of mature cells throughout the lifespan (1,2). Ironically, the BM niche is also the site for malignant hematopoiesis. Leukemia cells, akin to the invasive weed Parthenium hysterophorus that destroyed the habitat of native plant & animal species (3), are ruthless competitors and destroy the natural habitat of normal hematopoietic stem and progenitor cells by altering the behavior of supporting cells in the BM niche (4-6). Apart from leukemias, defects in hematopoiesis are frequently observed in different cancer patients with BM metastasis (7,8).
The endothelial cells are an important constituent in the BM niche apart from osteoblasts, adipocyte and several variants of perivascular stromal cells including the Sca1+ and CD146+ mesenchymal stromal cells (MSCs) we recently identified (9), nestin+ MSCs (10), leptin receptor expressing mesenchymal cells (11), and CXCL12 abundant reticular (CAR) cells (12,13). The endothelial cells secrete stem cell factor (SCF or KITL), CXCL12 and other growth factors important to HSC maintenance and regeneration (13,14). Studies using Tie2-cre transgene to target the BM endothelial cells revealed that the selective deletion of SCF or CXCL12 from endothelial cells resulted in depletion of primitive HSCs and/or loss of long-term repopulating capability (15). Recent studies have made significant progress using unique marker combinations to differentiate different endothelial cell types and this has allowed the determination that the arteriolar and sinusoidal vascular cells play opposing roles in supporting HSCs. Arterioles are located near the bone and maintain HSCs in a quiescent state, whereas sinusoids are found throughout the BM compartment and support proliferating HSCs (15). Kusumbe et al., using confocal imaging of BM microvasculature, showed that a functionally distinct population of vessels called H type endothelium (marked by high levels of endomucin) is essential for bone and vessel growth (16).
By using two photon imaging, Duarte and colleagues tracked acute myeloid leukemia (AML) progression in the mice calvarium BM niche. Different transgenic reporters were used to help them to track the interaction of infiltrating AML cells with other niche constituents in the real time as leukemia burden increases (17) (Figure 1). Unlike chronic myeloid leukemia (CML) and myelodysplastic syndrome (MDS), the leukemia stem cells (LSCs) of AML are very heterogenous and aren’t confined to CD34+CD38− fraction. Disease modeling has shown that even mature granulocyte/monocyte progenitors (GMPs) can also produce transplantable AML LSCs and results in AML development (18). In this study, the authors adopted a well-established MLL-AF9 driven murine AML model by transducing myeloid progenitors with retrovirus that carried MLL-AF9-GFP transgene and generated a transplantable syngeneic AML model. The advantage of this model is the ability to leave normal HSC niche unperturbed by myeloablative radiation or chemotherapy during transplantation. The secondary MLL-AF9 recipient mice developed full blown AML around 20–28 days, similar to patients with MLL rearranged leukemia.
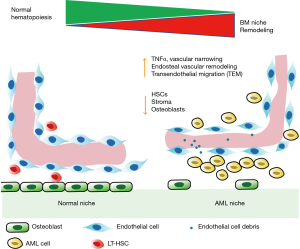
Using Flk1-GFP (endothelial specific reporter) mice, they showed that leukemia development in the mice moved the endothelial cells away from the endosteal surface and the vasculature were narrower compared to the control mice. AML cells bearing mice had higher trans-endothelial migration (TEM) of blasts which escaped from blood vessels due to increased vascular permeability in AML mice compared to the control mice. Similar to AML patients, angiogenesis was increased in the AML mice based on the increased sprouting from BM endothelial cells (19). However, the number of associated blood vessel were only significantly reduced in endosteal and metaphyseal regions. The vasculature in diaphysis were unchanged in the AML mice suggesting heterogeneity in the AML induced vascularity remodeling. Additionally, an increase in vessel oscillations and turbulent blood flow was observed due to intravascular clustering of AML cells that adhere to the endothelium and block blood flow within AML Infiltrated BM endothelium compared to control mice.
Besides changes to endosteal vasculatures, Duarte and colleagues also found dramatic reduction in stoma cells numbers in vivo, including the stroma surrounding blood vessels and adjacent to the bone compared to control mice. Further, there was a progressive decline in the number of long-term HSCs (LT-HSCs) and osteoblasts with leukemia progression. In AML burdened mice, gene set enrichment analysis (GSEA) comparing endosteal and central AML cells revealed that an abundance of inflammatory genes were enriched in endosteal AML cells. ELISA analysis of BM supernatant obtained from different regions of BM also demonstrated increased levels of inflammation cytokines TNF and CXCL2 in endosteal region, supporting a role for CXCL2 and TNF in remodeling of endosteal vessels. These results, in line with evidences from other leukemia mouse models, suggesting pro-inflammatory signals are key players in AML evolution and maintenance (20).
Lastly, in an attempt to normalize the diseased BM niche, Duarte and colleagues treated AML burden mice with deferoxamine (DFO), an iron chelator recently shown to expand the endosteal vasculature by upregulating HIF1a expression (16). While the DFO treated mice indeed show increased number of endosteal vessels, the DFO treatment didn’t have much effect on AML disease burden and animal survival. A recent study, using a xenograft model, similarly showed that the AML engraftment caused increased vascular permeability in the BM niche. Additionally, they found significantly higher levels of NOX4 and NOS3 genes in the BM endothelial cells of AML burdened mice. The increased nitric oxide (NO) production in the BM niche caused damage to the BM endothelium. Inhibition of NO production in BM niche by genetic or pharmacological methods not only restored the vascular permeability defects, it also enhanced normal HSC functions, improved drug delivery and animal survival (21). Lastly, using genetic models for Notch activation in endothelial cells, Ralf Adams’ group improved endosteal vessels regeneration and numbers in aged mice and enhanced the efficacy of drug delivery (22,23). These results supported the notion of niche restoration as additional therapeutic opportunities to improve on current leukemia treatments.
Overall, this study by Duarte and colleagues is in line with the current hypothesis that leukemia cells damage the BM components in the niche and diminish normal HSC niche to favor their own expansion. This study is also consistent with our recent published works showing that AML cells through their secreted exosomes could remodel endosteal BM niche promoting their own expansion, inhibits normal HSC functions and either normalization of endosteal niche by reducing leukemia induced DKK1 expression or leukemia specific exosomes secretion inhibition could rescue and enhance chemotherapy efficacy (24,25).
For effective leukemia treatment, this study adds to the growing research that suggests importance of restoration of the defective niche. Restoring damaged vascular microenvironment may increase chemotherapy delivery and increase treatment responses. Further, niche targeted therapy may inspire the design of innovative therapies that are not only shrinking/killing the proliferating blasts but also eliminating LSCs, a quiescent population responsible for disease relapses. This is an exciting study that provides a detailed mechanism of leukemic evolution and the possibility for future studies on microenvironment specific therapeutic targets.
Acknowledgements
Funding: Funding was provided by the American Cancer Society Grant 128766-RSG-15-162.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature 2014;505:327-34. [Crossref] [PubMed]
- Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med 2014;20:833-46. [Crossref] [PubMed]
- Oudhia P. Positive (inhibitory) allelopathic effects of Parthenium leaves on germination and seedling vigour of sunflower. Crop Research (Hisar) 2000;20:560-2.
- Sipkins DA, Wei X, Wu JW, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 2005;435:969-73. [Crossref] [PubMed]
- Colmone A, Amorim M, Pontier AL, et al. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science 2008;322:1861-5. [Crossref] [PubMed]
- Hawkins ED, Duarte D, Akinduro O, et al. T-cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. Nature 2016;538:518-22. [Crossref] [PubMed]
- Shiozawa Y, Pedersen EA, Havens AM, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 2011;121:1298-312. [Crossref] [PubMed]
- Wu WC, Sun HW, Chen HT, et al. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc Natl Acad Sci U S A 2014;111:4221-6. [Crossref] [PubMed]
- Hu X, Garcia M, Weng L, et al. Identification of a common mesenchymal stromal progenitor for the adult haematopoietic niche. Nat Commun 2016;7:13095. [Crossref] [PubMed]
- Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010;466:829-34. [Crossref] [PubMed]
- Zhou BO, Yue R, Murphy MM, et al. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 2014;15:154-68. [Crossref] [PubMed]
- Sugiyama T, Kohara H, Noda M, et al. Maintenance of the Hematopoietic Stem Cell Pool by CXCL12-CXCR4 Chemokine Signaling in Bone Marrow Stromal Cell Niches. Immunity 2006;25:977-88. [Crossref] [PubMed]
- Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013;495:231-5. [Crossref] [PubMed]
- Ding L, Saunders TL, Enikolopov G, et al. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 2012;481:457-62. [Crossref] [PubMed]
- Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013;502:637-43. [Crossref] [PubMed]
- Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014;507:323-8. [Crossref] [PubMed]
- Duarte D, Hawkins ED, Akinduro O, et al. Inhibition of Endosteal Vascular Niche Remodeling Rescues Hematopoietic Stem Cell Loss in AML. Cell Stem Cell 2018;22:64-77.e6. [Crossref] [PubMed]
- Krivtsov AV, Twomey D, Feng Z, et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature 2006;442:818-22. [Crossref] [PubMed]
- Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood 2000;95:309-13. [PubMed]
- Pietras EM. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood 2017;130:1693-8. [Crossref] [PubMed]
- Passaro D, Di Tullio A, Abarrategi A, et al. Increased vascular permeability in the bone marrow microenvironment contributes to disease progression and drug response in acute myeloid leukemia. Cancer Cell 2017;32:324-341.e6. [Crossref] [PubMed]
- Kusumbe AP, Ramasamy SK, Itkin T, et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 2016;532:380-4. [Crossref] [PubMed]
- Ramasamy SK, Kusumbe AP, Wang L, et al. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 2014;507:376-80. [Crossref] [PubMed]
- Kumar B, Garcia M, Weng L, et al. Acute myeloid leukemia transforms the bone marrow niche into a leukemia-permissive microenvironment through exosome secretion. Leukemia 2018;32:575-87. [Crossref] [PubMed]
- Kumar B, Garcia M, Murakami JL, et al. Exosome-mediated microenvironment dysregulation in leukemia. Biochim Biophys Acta 2016;1863:464-70. [Crossref] [PubMed]
Cite this article as: Kumar B, Chen CC. Acute myeloid leukemia remodels endosteal vascular niche into a leukemic niche. Stem Cell Investig 2018;5:34.


