Lung cancer stem cells—origin, characteristics and therapy
Introduction
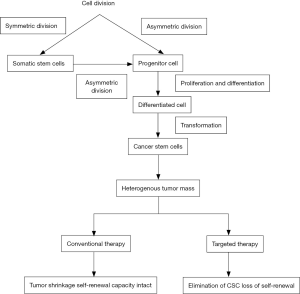
Lung cancer has remained the most common occurring cancer globally. Traditional methods like surgery, radiotherapy and chemotherapy are the treatment methods for lung cancer. Yet resistance to radiotherapy and chemotherapy, relapse in patients has always remained a challenging issue in lung cancer treatment. This resistance is attributed to a class of cells known as cancer stem cells (CSC) (Figure 1). CSCs constitute a unique subset of cells of tumor cells which indefinitely enable the growth of a malignant population of cells (1).
The CSC hypothesis is now the widely studied field in oncology. CSC hypothesis suggests that cell heterogeneity within a tumor is due to the presence of a small subpopulation of cells that display the properties of normal somatic stem cells (2). Several attributes of somatic stem cells and CSCs are similar such as self-renewal capacity, the ability to differentiate and produce multi-lineage progeny that are tumorigenic and non-tumorigenic, the capacity to establish and maintain tumors, and flexibility in attaining these characteristics (3). The characteristic properties of lung CSCs are characterized by several studies as; self-renewal, multipotent differentiation, tumorigenic potential, expression of stem cell markers, increased invasiveness, proliferation as tumor spheres, chemo resistance, radio resistance to hypoxia, resistance to apoptosis, quiescence (4).
The intrinsic resistance of these CSCs to therapy is the result of increased telomere length (5), activation of anti-apoptotic pathways, increased membrane transporter activity (6) and the ability to migrate and metastasize (7). Lung CSCs display longer telomeres than non CSC counterparts. Serrano et al. (8) showed that treatment with the specific telomerase inhibitor, MST312, had a strong and preferential anti-proliferative effect on the lung CSC population in vitro and in vivo. ATP-binding cassette (ABC) transporters, such as p-glycoprotein and multidrug resistant associated protein (MRP1), are membrane transporters that can pump structurally unrelated small molecules, such as cytotoxic chemotherapeutic drugs out of the cell. Normal stem cells and lung CSCs express high levels of ABC transporters resulting in low intracellular drug concentrations (9). Studies indicate the high expression of ABCG2 gene in lung CSCs but are switched off in most terminally differentiated progeny (10).
CSCs origin
The origin of CSCs is still under question and the different theories proposed for origin of CSCs include cell fusion, horizontal gene transfer, cell microenvironment and mutations (11,12).
Cell theory proposes that CSCs could be originating due to cell fusion between tumor cells and bone marrow derived cells that come from a tissue suffering chronic inflammation (13). This theory had been supported by findings of an in vivo animal model study done by Houghton et al. (14). The study emphasized that bone marrow-derived cells (BMDC) undergo metaplasia and dysplasia eventually leading to cancer. Mouse bone marrow cells can also undergo spontaneous cell fusion and take up the phenotype of the other cell (15). In addition, researchers suggest that cell fusion could also be the source of recurrent CSCs. These are basically the cells that originate after the first set of therapy and trigger cancer. It is also known that the tumor tissue after therapy consisted of fusogenic cells such as CSCs, tumor cells, macrophages/monocytes and BMDC (16). Similar studies on humans proved that the BMDC from donor and tumor cells of recipient fused together thereby increasing the malignancy of the tumor (17). Moreover recent studies on human cancer demonstrated that BMDC/tumor cell hybrid creates cells that are resistant to radiotherapy and have enriched cell repair mechanisms (18). Though these studies do not directly provide evidence for origin of stem cells, it is clear that cell fusion is highly likely to give rise to CSCs.
Horizontal gene transfer (HGT) which played a major role in dispersion of antibiotic resistance has also been discovered to be involved in cancer progression (19). In vivo studies have proved that apoptotic cells transfer their genes to the recipient cells during the process of phagocytosis (20). Another theory arose from the fact that cell-free DNA is capable of circulating in the eukaryotes until being taken by a recipient cell. Animal model studies demonstrate colon cancer progression through horizontal gene transfer in immunocompetent mice (21,22).
CSCs can accumulate more genetic mutations that boost the cancer progression and resistance through HGT. Mutations are well known for causing cancer through inheritance, environmental carcinogens or DNA replication errors (23). Merkle et al. demonstrated that human stem cells are capable of taking up large copy number variants. Five out of the 140 cell lines they tested had six mutations that are dominant in Tp53 gene. Tp53 gene was responsible for suppressing tumor growth in humans. The mutant allele was observed to escalate with the passage number revealing that stem cells accept mutations that confer them with growing abilities. This also necessitates the need to genetically analyze the stem cells before using them for therapies or clinical applications (24).
Microenvironment of a cell is made of factors that influence the cell conditions and behavior either directly or indirectly. Some of these factors include the extracellular matrix (ECM), hormones, neighboring cells and forces acting as a result of movement of the host (25). The factors contributing to cancer progression are mostly common in several different cancers. The stem cell micro environmental control plays a major role in maintaining the plasticity of the cell. Any errors in the control can lead to dedifferentiation of stem cells thereby causing cancer. A study led by Kidd et al. showed that mesenchymal stem cells (MSC) exhibit tropism in response to a cytokine environment. Interestingly, such cytokine environments are seen in tumor growth areas which in turn allow the incorporation of MSCs in the cancerous region (26). Moreover MSCs are basically derived from bone marrow which implies that cell fusion and microenvironment could synergistically act to develop cancers (27). Moreover it has also been reported that Helicobacter felis infection in mice stimulates the flow of bone marrow stem cells into the stomach. Due to lineage difference, it is suggested that this influx could lead to stomach cancer (12). Additionally studies have also proved that inflammatory microenvironments support the pre-cancerous lesion growth and tumor genesis. The cancer microenvironment is constituted of a thicker ECM than that found in the normal one. Tumor microenvironments holding different factors can also indirectly facilitate the tumor heterogeneity and resistance to chemotherapy (28,29).
Another new theory on origin of CSCs is based on autoreactive T-cells. The researchers suggested that CSCs emerge from autoreactive T-cells that are not completely killed by a weak immune system (30). This brings about a new approach in cancer therapy where we need to focus on bolstering the immune system rather than weakening it.
The origin of lung CSCs has been traced back to cells on specific anatomical sites on lungs. The basal cells of proximal airway (trachea and bronchi) associated with squamous cell carcinoma exhibiting stem cell like behavior was found to over express keratin. Clara cells and pulmonary neuroendocrine cells (PNEC) associated with small cell lung cancer exhibiting stemness had altered expression of secretoglobin and calcitonin related peptide. Lung adenocarcinoma and bronchoalveolar carcinoma has been associated with stem cells from the bronchoalveolar duct junction region. Studies on mouse models demonstrated the association of Matrixmetalloproteinase-10 (MMP10) with the maintenance and tumorigenicity of lung CSC (31,32).
Lung CSC markers
Though the current knowledge on lung CSC biology is limited, a number of CSC markers have been identified and studied. These CSC markers are associated with resistance to anti-cancer therapies. Few of them include; include aldehyde dehydrogenase (ALDH1), CD133, side population (Hoechst-negative), CD44, CD87 and CD117. The identification of unique lung CSC markers still remains challenging mainly due to the intratumoral heterogeneity and high degree of plasticity that can cause instability of the CSC phenotype and reversion of cell surface markers.
CD133
CD133 is the most frequently demonstrated CSC marker. It is a cell surface glycoprotein that consists of five transmembrane domains and two large glycosylated extracellular loops. It was considered a stem cell marker only in the hematopoietic system and the nervous system. The membrane antigen also is a marker for tumorigenic cells in certain other solid tumors. CD133 positive cells displayed higher ability of self-renewal, tumor initiation and drug resistance (33). Chen et al. demonstrated that Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive Cells. Oct-4 expression is normally found in totipotent and pluripotent stem cells of pregastrulation embryos. This implicates Oct-4 play a crucial role in maintaining cancer stem-like and chemo, radioresistant properties in lung cancer derived CD133+ cells (34). Cui et al. investigated various human lung cancer cell lines A549, H157, H226, Calu-1, H292 and H446. The results of real-time PCR analysis after chemotherapy drug selection and the fluorescence-activated cell sorting analysis showed that CD133 only functioned as a marker in the small cell lung cancer line H446 (35).
CD90
CD90 expression was primarily identified in murine breast CSCs in liver tumourogenesis. CD90 (Thy-1) is a 25–37 kDa glycosylphosphatidylinositol (GPI)-anchored glycoprotein expressed mainly in leukocytes and is involved in cell-cell and cell-matrix interactions. Yan et al. explored experimentally the use of CD90 as a marker for identification of lung CSCs (36). The previous studies of these workers demonstrated stronger proliferation and self-renewal abilities, and higher levels expression of the stem cell markers Sox2 and Oct4 in A549 and H446 cell lines.
ALDH1
The ALDH family is cytosolic isoenzyme catalyzing oxidation of intracellular aldehydes. Jiang et al. were the first to isolate ALDH1-positive cells from human lung cancer cell lines and showed ALDH1-positive cancer cells exhibited the important CSC properties: in vitro self-renewal, differentiation, and multidrug resistance capacities. Furthermore, they analyzed ALDH1 expression in lung tissues from three different populations of patients with lung cancer, and reported that increased ALDH1 protein levels were positively associated with stage and grade of the tumors and inversely related to the patients’ survival (37).
Another stem cell marker CD44 is a multifunctional class 1 transmembrane glycoprotein mediating complex functions is found associated with breast and prostate cancers. CD44, alone or in combination with other putative CSC markers such as CD24 is used in identification of tumors (38,39). Leung showed that CD44+ cells are enriched for tumor propagating capacity and CD44 is a potential CSC marker of NSCLC cell lines (40).
Studies report a small portion of CSC cells can be enriched in the side population cells (SP) after fluorescence activated cell sorting. The activation was due to ABC transporters such as ABCG2 activation in this group of cells which cannot be stained with Hoechst 33342 in comparison to cells treated with the pump inhibitor verapamil (41). SP cells possess higher efficiency and capacity of tumor-sphere formation. SP cells showed increased resistance to cisplatin, gemcitabine and Vinorelbine compared with the non-SP group (42-44). ABCG2 has been found to play a major role in the multidrug resistance phenotype, and elevated ABCG2 levels have been found in CSC cells in NSCLC. It has also been found that patients with the dual expression of CD133 and ABCG2 have a relative higher risk of tumor recurrence (45).
Signaling pathways
Hh pathway, Wnt pathway and Notch pathway which regulate proliferation and differentiation during embryogenesis are associated with CSC self-renewal also. The Hedgehog (HH) signaling pathway plays a key role in regulating morphogenesis, homeostasis and repair of stem cells in the adult human body. This pathway was found to be activated in both types of lung cancer—small cell and non-small cell lung cancer (46). It was discovered that HH pathway can increase the chemoresistance thereby causing failures of chemotherapy in lung cancers. Mutations caused in the HH pathway provide way to activation and advancement of tumorigenic pathway and CSCs eventually leading to cancer (47). The HH pathway involves three ligands namely Sonic Hedgehog (SHH), Desert Hedgehog (DHH) and Indian Hedgehog (IHH) that have various spatial and temporal expression levels. These ligands can also function as mitogens and promote cell division and differentiation (48). The main receptor of the ligands is the Patched receptor which is expressed near the source of the HH signals (49). The Patched receptor represses the activity of another transmembrane protein called Smoothened (Smo) when there are no HH signals. However when one of the three ligands bind to the Patched receptor, SMO accumulates and activates the transcriptional factors of GLI family which will then move into the nucleus to activate the HH target genes .
Signaling by the Wnt pathway is exceedingly complex in mammalian cells. The canonical pathway, Wnt ligands bind to a cell surface receptor complex causing the phosphorylation of disheveled family proteins (Dvl). The Dvl then activates glycogen synthase kinase 3 (GSK-3) and casein kinase 1 (CK1), which mediates the degradation of β-catenin molecules, resulting in the accumulation of β-catenin in the cytoplasm. Some β-catenin is able to enter the nucleus and interact with transcription factor/lymphoid enhancer-binding factor 1 (TCF/LEF) family transcription factors to promote specific gene expression. It has been shown that Wnt1 and Wnt2 are over expressed in NSCLC cell lines and primary tumors (50,51).
The Notch pathway is an evolutionarily conserved signaling system which plays diverse roles in normal tissue development and homeostasis. In humans the Notch pathway comprises four receptors (Notch1–4) and five ligands, JAG1, JAG2, DLL1, DLL3, and DLL4. Numerous lines of evidence link the Notch pathway to cancer, for example, activating mutations in Notch1 are detected in T-cell leukemias and components of the pathway have been linked to the progression and metastasis of solid tumors (52-54).
Therapeutic approaches with lung CSCs
Being responsible for caner initiation and relapse, several therapies consider CSCs as key targets for cancer treatment (55). Studies suggest that since CSCs promote both primary and metastatic growth, targeting and destroying them should be the main goal for a successful therapy (56). A proper treatment of CSCs requires that perfect identification of type of CSC involved. This has been the most challenging part so far in the treatments targeting CSCs. Various surface markers for CSCs that are proteins precisely expressed on their surface, including receptors and antigens are now the targets for therapeutic studies (57). The technique used to screen for the surface markers is fluorescence assisted cell sorting (FACS) (58). By using a multiparametric cell sorter, one can also screen for other cellular features that are unique to CSCs. It can also identify rare small cell populations that are the leading cause for the aggressive form of lung cancer (59). The well-known candidate surface markers for CSCs are CD 133, CD24 and CD44. Based on the organ where the cancer begins, these markers might be expressed differently, along with a few other markers. For instance CSCs in breast cancer are screened for CD44 and/or CD24 combined with ALDH1 (60). A recent review by Katarzyna suggests that MET tyrosine kinase receptors are potential CSC markers that can be used as effective targets as they play a major role in several types of cancers by supporting the CSCs with their versatile phenotype and therapy resistance (61). Another important cell surface marker is the CD90 that are highly expressed in lung cancer cells (62). A recent study discovered that CD90 is specifically expressed by CSCs of insulinomas which makes it an ideal therapeutic target for treatment of insulinoma (63). Another effective target could be CD47, a transmembrane protein which is seen in almost all solid tumors .Novel CSC based treatment will reach the above mentioned targets by using their respective antibodies against them (64).
Although a great progress has been made in identification of CSCs with surface markers, it is important to understand that all CSCs do not express the surface markers and in some cases the non-CSCs also express surface markers. Moreover, CSCs of same origin can express different surface markers making the isolation complicated. This implies that surface markers based identification is basically isolating the CSC-rich cell population and not exactly the individual CSCs (58). Thus, additional in vitro and in vivo assays that affirm the self-propagating and self-renewal property of CSCs might be necessary to certainly isolate the CSCs. However, CSCs exhibit phenotypic instability once they are outside the solid tissue which makes the precise identification hard to achieve (65).
Another way of identifying CSCs is through their cell signaling pathways. This approach seems to be a promising one as many types of CSCs share the same signaling pathways to initiate cancer in the host. The PI3K/Akt and Hedgehog pathways are some of the common target pathways that are used by CSCs to activate the subsistence and propagation mechanisms of tumor cells. This pathway is under constitutive expression in 30–40% of human cancers. Rather than altering the entire pathway, scientists are focused towards knocking down one important enzyme/factor in the pathway that will potentially shut down the whole process. Studies found that HH inhibitors can also suppress cancer-associated fibroblasts that regulate the function and motility of human lung CSCs (66). This could be a novel strategy towards the treatment of lung and breast cancer (67).
One of the key factors that have become an effective target is phosphatidylinositol 3-kinase (PI3K). PI3K has been reported to acquire cancerous mutations in several types of human cancer (68). Another major target is the class of rapamycin. Rapamycin inhibitors developed by researchers can knock down the M TOR portion of the pathway which regulates the metabolism, protein translation and cell growth (69). Recent studies recommend the use of alpha fetoprotein, a product of activated PI3K/Akt pathway as a potential cancer target. This protein is reported to induce the reprogramming of liver non-CSCs and CSCs to form malignant tumors (70). Another interesting target is Wasp Interacting Protein (WIP) which is regulated by PI3K/Akt-based integrin/receptor recycling pathway. WIP is involved in driving the oncogenic activity of the mutant p53 gene, which is the major tumor suppressor gene in its wild form (71).
Effective inhibition of CSCs is also observed by targeting their efflux pumps and tumor micro environment. The major drug transporter P-glycoprotein (Pgp) is now regarded as a valuable target in cancer therapy as it is responsible for multi drug resistance in cancer. Pgp inhibitors used in combination with conventional cancer drugs seems to be a less toxic approach as Pgps are specifically expressed by cancer cells and not the normal cells (72).
Lung cancer cells displaying CD133 marker were also associated with a higher expression of Oct-4 which helps them in self renewal and metastasis. Recent works utilize small interfering RNA to knock down the Oct-4 gene expression in human lung cancer cells and showed that CD133+ cells lost their ability to form spheres and differentiated back to CD133− cells. In addition, the treatment also enhanced the apoptotic activity of caspase 3 and poly (ADP-ribose) polymerase thereby making the cancer cells more susceptible to chemotherapeutic drugs. In case of lung CSCs, the Oct-4 is predicted to act by Tcl1/Akt1 pathway that inhibits apoptosis. Thus targeting the Oct-4 can result in the programmed cell death of lung CSCs. This has been proven in vitro using murine Lewis lung carcinoma cells (73). An endogenous mitochondrial anti-oxidant called alpha lipoic acid was also recently found to suppress Oct-4 in human lung cancer cells. It reduced the phenotype of lung CSCs such as anchorage-independent growth and three dimensional sphere formations by diminution of the cellular proteins β-catenin and Oct-4. This was achieved by affecting the amount of active (phosphorylated) Akt (74). Thus Oct-4 could be a potential and novel target for enhancing the treatment of lung cancer. The function of miR-34a in regulating NSCLC cell behavior has not been extensively studied. Shi et al. showed that transfection of synthetic miR-34a in three NSCLC cell lines, A549, H460, and H1299, inhibited tumor regeneration in vivo. Furthermore, the lentiviral vector-mediated overexpression of miR-34a in purified CD44hi H460 cells also inhibited tumor outgrowth. On the other hand, expression of miR-34a antisense oligos in the CD44lo H460 cells promoted tumor development. These studies showed that miR-34a is a negative regulator of the tumorigenic properties of NSCLC cells and CD44hi lung CSCs, and establishes a strong rationale for developing miR-34a as a novel therapeutic agent against NSCLC (75).
Alternate strategy would be targeting the CSCs niche with integrin. Integrin is the primary receptor that is involved in cell-matrix adhesion and has a profound impact on the ability of CSCs to survive in specific locations. α6β1 integrin has been implicated in the function of breast and other CSCs yet little is known about its regulation and relationship to mechanisms involved in the genesis of CSCs. Goel et al. reported that a CD44(high)/CD24(low) population, enriched for CSCs, is comprised of distinct epithelial and mesenchymal populations that differ in expression of the two α6 α6A and α6B. α6Bβ1 expression defines the mesenchymal population and is necessary for CSC function, a function that cannot be executed by α6A integrins (76). Yet, specific integrins that is liable to promote stemness, drug resistance, and metastasis of CSCs are yet to be determined (77). Studies report a few integrin α2-binding agents that have been made up to reduce the proliferation capability of CSCs (78).
Conclusions
Lung cancer therapy has become most complicated due to the relapse and recurrence of the disease. Of recent many studies are concentrated on a class of cells—CSCs, which are responsible for conferring the ability of tumor regeneration indefinitely. This review focused on the origin, characteristics of CSCs, lung CSC markers, role of the signaling pathways and novel therapeutic approaches. A great deal of knowledge is essential on the biology and gene expression of these stem cells for targeted therapy. This targeted therapy on CSCs could be incorporated along with traditional therapy which would augment the effectiveness.
Acknowledgements
The authors would like to acknowledge the support provided by Sathyabama Institute of Science & Technology.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- MacDonagh L, Gray SG, Breen E, et al. Lung cancer stem cells: The root of resistance. Cancer Lett 2016;372:147-56. [Crossref] [PubMed]
- Morrison BJ, Morris JC, Steel JC. Lung cancer-initiating cells: a novel target for cancer therapy. Target Oncol 2013;8:159-72. [Crossref] [PubMed]
- Rivera C, Rivera S, Loriot Y, et al. Lung cancer stem cell: new insights on experimental models and preclinical data. J Oncol 2011;2011:549181. [Crossref] [PubMed]
- Hiyama E, Hiyama K. Telomere and telomerase in stem cells. Br J Cancer 2007;96:1020-4. [Crossref] [PubMed]
- Zhou S, Schuetz JD, Bunting KD, et al. The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 2001;7:1028-34. [Crossref] [PubMed]
- Brabletz T, Jung A, Spaderna S, et al. Opinion: migrating cancer stem cells - an integrated concept of malignant tumour progression. Nat Rev Cancer 2005;5:744-9. [Crossref] [PubMed]
- Serrano D, Bleau AM, Fernandez-Garcia I, et al. Inhibition of telomerase activity preferentially targets aldehyde dehydrogenase-positive cancer stem-like cells in lung cancer. Mol Cancer 2011;10:96. [Crossref] [PubMed]
- Chen LS, Wang AX, Dong B, et al. A new prospect in cancer therapy: targeting cancer stem cells to eradicate cancer. Chin J Cancer 2012;31:564-72. [Crossref] [PubMed]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005;5:275-84. [Crossref] [PubMed]
- Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res 2006;66:4553-7. [Crossref] [PubMed]
- Bu Y, Cao D. The origin of cancer stem cells. Front Biosci (Schol Ed) 2012;4:819-30. [PubMed]
- Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow-derived cells. Science 2004;306:1568-71. [Crossref] [PubMed]
- Dittmar T, Nagler C, Niggemann B, et al. The dark side of stem cells: triggering cancer progression by cell fusion. Curr Mol Med 2013;13:735-50. [Crossref] [PubMed]
- Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002;416:542-5. [Crossref] [PubMed]
- Dittmar T, Nagler C, Schwitalla S, et al. Recurrence cancer stem cells--made by cell fusion? Med Hypotheses 2009;73:542-7. [Crossref] [PubMed]
- Pawelek JM. Fusion of bone marrow-derived cells with cancer cells: metastasis as a secondary disease in cancer. Chin J Cancer 2014;33:133-9. [Crossref] [PubMed]
- Lindström A, Midtbö K, Arnesson LG, et al. Fusion between M2-macrophages and cancer cells results in a subpopulation of radioresistant cells with enhanced DNA-repair capacity. Oncotarget 2017;8:51370-86. [Crossref] [PubMed]
- Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 2010;74:417-33. [Crossref] [PubMed]
- Bergsmedh A, Szeles A, Henriksson M, et al. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc Natl Acad Sci U S A 2001;98:6407-11. [Crossref] [PubMed]
- Trejo-Becerril C, Pérez-Cárdenas E, Taja-Chayeb L, et al. Cancer progression mediated by horizontal gene transfer in an in vivo model. PLoS One 2012;7:e52754. [Crossref] [PubMed]
- Shaw JA, Page K, Blighe K, et al. Genomic analysis of circulating cell-free DNA infers breast cancer dormancy. Genome Res 2012;22:220-31. [Crossref] [PubMed]
- Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science 2017;355:1330-4. [Crossref] [PubMed]
- Merkle FT, Ghosh S, Kamitaki N, et al. Human pluripotent stem cells recurrently acquire and expand dominant negative P53 mutations. Nature 2017;545:229-233. [Crossref] [PubMed]
- Barthes J, Özçelik H, Hindié M, et al. Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: the recent advances. Biomed Res Int 2014;2014:921905. [Crossref] [PubMed]
- Kidd S, Spaeth E, Dembinski JL, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 2009;27:2614-23. [Crossref] [PubMed]
- Gregory CA, Prockop DJ, Spees JL. Non-hematopoietic bone marrow stem cells: molecular control of expansion and differentiation. Exp Cell Res 2005;306:330-5. [Crossref] [PubMed]
- Prasetyanti PR, Medema JP. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer 2017;16:41. [Crossref] [PubMed]
- Relation T, Dominici M, Horwitz EM. Concise Review: An (Im)Penetrable Shield: How the Tumor Microenvironment Protects Cancer Stem Cells. Stem Cells 2017;35:1123-30. [Crossref] [PubMed]
- Grandics P. The cancer stem cell: evidence for its origin as an injured autoreactive T cell. Mol Cancer 2006;5:6. [Crossref] [PubMed]
- Salama R, Tang J, Gadgeel SM, et al. Lung Cancer Stem Cells: Current Progress and Future Perspectives. J Stem Cell Res Ther 2012;2:1. [PubMed]
- Hardavella G, George R, Sethi T. Lung cancer stem cells-characteristics, phenotype. Transl Lung Cancer Res 2016;5:272-9. [Crossref] [PubMed]
- Herpel E, Jensen K, Muley T, et al. The cancer stem cell antigens CD133, BCRP1/ABCG2 and CD117/c-KIT are not associated with prognosis in resected early-stage non-small cell lung cancer. Anticancer Res 2011;31:4491-500. [PubMed]
- Chen YC, Hsu HS, Chen YW, et al. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS One 2008;3:e2637. [Crossref] [PubMed]
- Cui F, Wang J, Chen D, et al. CD133 is a temporary marker of cancer stem cells in small cell lung cancer, but not in non-small cell lung cancer. Oncol Rep 2011;25:701-8. [PubMed]
- Yan X, Luo H, Zhou X, et al. Identification of CD90 as a marker for lung cancer stem cells in A549 and H446 cell lines. Oncol Rep 2013;30:2733-40. [Crossref] [PubMed]
- Jiang F, Qiu Q, Khanna A, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res 2009;7:330-8. [Crossref] [PubMed]
- Naor D, Wallach-Dayan SB, Zahalka MA, et al. Involvement of CD44, a molecule with a thousand faces, in cancer dissemination. Semin Cancer Biol 2008;18:260-7. [Crossref] [PubMed]
- Hurt EM, Kawasaki BT, Klarmann GJ, et al. CD44+ CD24(-) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer 2008;98:756-65. [Crossref] [PubMed]
- Leung EL, Fiscus RR, Tung JW, et al. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS One 2010;5:e14062. [Crossref] [PubMed]
- Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 1996;183:1797-806. [Crossref] [PubMed]
- Hu L, McArthur C, Jaffe RB. Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. Br J Cancer 2010;102:1276-83. [Crossref] [PubMed]
- Wang YH, Li F, Luo B, et al. A side population of cells from a human pancreatic carcinoma cell line harbors cancer stem cell characteristics. Neoplasma 2009;56:371-8. [Crossref] [PubMed]
- Liu Y, Lu WL, Guo J, et al. A potential target associated with both cancer and cancer stem cells: a combination therapy for eradication of breast cancer using vinorelbine stealthy liposomes plus parthenolide stealthy liposomes. J Control Release 2008;129:18-25. [Crossref] [PubMed]
- Li F, Zeng H, Ying K. The combination of stem cell markers CD133 and ABCG2 predicts relapse in stage I non-small cell lung carcinomas. Med Oncol 2011;28:1458-62. [Crossref] [PubMed]
- Zakaria N, Satar NA, Abu Halim NH, et al. Targeting Lung Cancer Stem Cells: Research and Clinical Impacts. Front Oncol 2017;7:80. [Crossref] [PubMed]
- Abe Y, Tanaka N. The Hedgehog Signaling Networks in Lung Cancer: The Mechanisms and Roles in Tumor Progression and Implications for Cancer Therapy. Biomed Res Int 2016;2016:7969286. [Crossref] [PubMed]
- Ng JM, Curran T. The Hedgehog's tale: developing strategies for targeting cancer. Nat Rev Cancer 2011;11:493-501. [Crossref] [PubMed]
- Quijada L, Callejo A, Torroja C, et al. The Patched Receptor: Switching On/Off the Hedgehog Signaling Pathway. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
- He B, Barg RN, You L, et al. Wnt signaling in stem cells and non-small-cell lung cancer. Clin Lung Cancer 2005;7:54-60. [Crossref] [PubMed]
- Uematsu K, He B, You L, et al. Activation of the Wnt pathway in non small cell lung cancer: evidence of dishevelled overexpression. Oncogene 2003;22:7218-21. [Crossref] [PubMed]
- Hori K, Sen A, Artavanis-Tsakonas S. Notch signaling at a glance. J Cell Sci 2013;126:2135-40. [Crossref] [PubMed]
- Palermo R, Checquolo S, Bellavia D, et al. The molecular basis of notch signaling regulation: a complex simplicity. Curr Mol Med 2014;14:34-44. [Crossref] [PubMed]
- Alketbi A, Attoub S. Notch Signaling in Cancer: Rationale and Strategies for Targeting. Curr Cancer Drug Targets 2015;15:364-74. [Crossref] [PubMed]
- Dragu DL, Necula LG, Bleotu C, et al. Therapies targeting cancer stem cells: Current trends and future challenges. World J Stem Cells 2015;7:1185-201. [PubMed]
- de Sousa e Melo F, Kurtova AV, Harnoss JM, et al. A distinct role for Lgr5+ stem cells in primary and metastatic colon cancer. Nature 2017;543:676-680. [Crossref] [PubMed]
- Gopalan V, Islam F, Lam AK. Surface Markers for the Identification of Cancer Stem Cells. Methods Mol Biol 2018;1692:17-29. [Crossref] [PubMed]
- Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin 2013;34:732-40. [Crossref] [PubMed]
- Greve B, Kelsch R, Spaniol K, et al. Flow cytometry in cancer stem cell analysis and separation. Cytometry A 2012;81:284-93. [Crossref] [PubMed]
- Horimoto Y, Arakawa A, Sasahara N, et al. Combination of Cancer Stem Cell Markers CD44 and CD24 Is Superior to ALDH1 as a Prognostic Indicator in Breast Cancer Patients with Distant Metastases. PLoS One 2016;11:e0165253. [Crossref] [PubMed]
- Miekus K. The Met tyrosine kinase receptor as a therapeutic target and a potential cancer stem cell factor responsible for therapy resistance Oncol Rep 2017;37:647-56. (Review). [Crossref] [PubMed]
- Leon G, MacDonagh L, Finn SP, et al. Cancer stem cells in drug resistant lung cancer: Targeting cell surface markers and signaling pathways. Pharmacol Ther 2016;158:71-90. [Crossref] [PubMed]
- Buishand FO, Arkesteijn GJ, Feenstra LR, et al. Identification of CD90 as Putative Cancer Stem Cell Marker and Therapeutic Target in Insulinomas. Stem Cells Dev 2016;25:826-35. [Crossref] [PubMed]
- Clayton S, Mousa SA. Therapeutics formulated to target cancer stem cells: Is it in our future? Cancer Cell Int 2011;11:7. [Crossref] [PubMed]
- Witt AE, Lee CW, Lee TI, et al. Identification of a cancer stem cell-specific function for the histone deacetylases, HDAC1 and HDAC7, in breast and ovarian cancer. Oncogene 2017;36:1707-1720. [Crossref] [PubMed]
- Kim SH, Choe C, Shin YS, et al. Human lung cancer-associated fibroblasts enhance motility of non-small cell lung cancer cells in co-culture. Anticancer Res 2013;33:2001-9. [PubMed]
- Valenti G, Quinn HM, Heynen GJ, et al. Cancer Stem Cells Regulate Cancer-Associated Fibroblasts via Activation of Hedgehog Signaling in Mammary Gland Tumors. Cancer Res 2017;77:2134-47. [Crossref] [PubMed]
- Martelli AM, Evangelisti C, Chiarini F, et al. Targeting the PI3K/AKT/mTOR signaling network in acute myelogenous leukemia. Expert Opin Investig Drugs 2009;18:1333-49. [Crossref] [PubMed]
- Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol 2010;28:1075-83. [Crossref] [PubMed]
- Zhu M, Li W, Lu Y, et al. HBx drives alpha fetoprotein expression to promote initiation of liver cancer stem cells through activating PI3K/AKT signal pathway. Int J Cancer 2017;140:1346-55. [Crossref] [PubMed]
- Escoll M, Gargini R, Cuadrado A, et al. Mutant p53 oncogenic functions in cancer stem cells are regulated by WIP through YAP/TAZ. Oncogene 2017;36:3515-27. [Crossref] [PubMed]
- Lehne G. P-glycoprotein as a drug target in the treatment of multidrug resistant cancer. Curr Drug Targets 2000;1:85-99. [Crossref] [PubMed]
- Hu T, Liu S, Breiter DR, et al. Octamer 4 small interfering RNA results in cancer stem cell-like cell apoptosis. Cancer Res 2008;68:6533-40. [Crossref] [PubMed]
- Phiboonchaiyanan PP, Chanvorachote P. Suppression of a cancer stem-like phenotype mediated by alpha-lipoic acid in human lung cancer cells through down-regulation of β-catenin and Oct-4. Cell Oncol (Dordr) 2017;40:497-510. [Crossref] [PubMed]
- Shi Y, Liu C, Liu X, et al. The microRNA miR-34a inhibits non-small cell lung cancer (NSCLC) growth and the CD44hi stem-like NSCLC cells. PLoS One 2014;9:e90022. [Crossref] [PubMed]
- Goel HL, Gritsko T, Pursell B, et al. Regulated splicing of the α6 integrin cytoplasmic domain determines the fate of breast cancer stem cells. Cell Rep 2014;7:747-61. [Crossref] [PubMed]
- Seguin L, Desgrosellier JS, Weis SM, et al. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol 2015;25:234-40. [Crossref] [PubMed]
- Weiss SJ, Dudley DT. Integrin Alpha-2 Binding Agents and Use Thereof to Inhibit Cancer Cell Proliferation. 2015. Google patent US 8975029 B2.
Cite this article as: Prabavathy D, Swarnalatha Y, Ramadoss N. Lung cancer stem cells—origin, characteristics and therapy. Stem Cell Investig 2018;5:6.