Engineering of extracellular vesicles as drug delivery vehicles
Introduction
A number of intercellular communication systems that deliver biological signals from cells to cells are discovered; these includes soluble factors (hormones, cytokines and growth factors and chemical messengers) and extracellular vesicles (EVs). First described by Trams et al. (1), EVs have been successfully isolated from different extracellular fluids including blood (2), urine (3), cerebrospinal fluid (4), breast milk (5), saliva (6) and even in culture supernatant (7).
They can be classified as exosomes, microvesicles (MV) and apoptotic bodies based on their biogenesis and/or size (8) (Figure 1). Exosomes, also known as nanosphere (size of 40 to 150 nm in diameter) are produced by invagination of endosomal membranes to form multivesicular bodies (MVBs) in endosomes and secreted by fusion of these vesicles with plasma membrane (9). Due to their subcellular origin, exosomes contain endosomal membrane proteins, membrane transport and fusion proteins (GTPases, Annexins and flotillin), tetraspanin proteins (CD63, CD81, CD82, CD53, and CD37), heat shock proteins, proteins associated with lipid rafts, including glycosylphosphatidylinositol-anchored proteins (10,11), and proteins involved in MVB biogenesis (Alix and TSG101) (12). Exosomes are rich in glycosphingolipids, cholesterol, phosphatidylserine and ceramide in the lipid bilayer (13) that accounts for their unique rigidity. Additional components of EVs include mRNAs, micro RNA (miRNA) and non-coding RNAs (14).
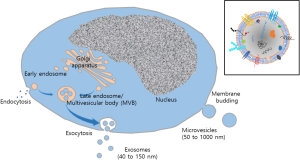
Microvesicles, also known as microparticles and ectosomes, are of 50 to 1,000 nm in diameter, produced by direct budding of plasma membrane and thus carry membrane proteins and lipids of plasma membrane from donor cells (15). Although a number of proteins may be used as MV markers, definitive marker(s) for MVs has not been identified. MVs are rich in phosphatidylserine on the outer leaflet (16). The release of MV shares similarities with viral release in terms of structural features and outward budding process (17). Biological molecules of the cargo include proteins (enzymes, growth factors, growth factor receptors, cytokines and adhesion molecules) (15) and nucleic acids, (mRNA, miRNA and ncRNA) (18). There is no standardized protocol for MV isolation available, many researchers utilize combination of differential centrifugation with sucrose gradient ultracentrifugation, size exclusion chromatography or immunoaffinity column in order to exclude cellular debris, exosome fraction and other subcellular particles during isolation (19). In contrast to exosomes or MV that are derived from heathy cells, apoptotic bodies (also known as apoptotic blebs, or apoptotic bodies) are released from apoptotic cells. Apoptotic bodies have a size ranging from 500 to 5,000 nm and contain subcellular contents (organelles), deoxyribonucleic acid (DNA), ribonucleic acid (RNA) and histone proteins. Currently, major research interest in EVs for drug delivery is focused primarily on exosomes and MV.
EVs are produced by virtually all species of living organisms and carrying biological signals that can influence behaviors of recipient cells implying their important roles in the development and function of tissues and organs. Unlike soluble factors, membrane-bound EVs can carry multiple biological information at once including proteins, nucleic acids (14,20) and lipids that can be shared between cells. Once secreted from donor cells, circulating EVs via biological fluids can be taken up by local or distant target cells and deliver the contents of the vesicles to target cells. While exosomes and/or MV can be isolated by single or combinations of different methods, including sedimentation by ultracentrifugation, density gradient ultracentrifugation, antibody-based separation, ultrafiltration, size exclusion chromatography, high performance liquid chromatography and fluorescent-activated cell sorter (21,22), a clear distinction of exosomes from MV in regards to their size, morphology, density, composition and physiological function is a challenging project; therefore, we will refer to EVs as a collective terms for both exosomes and MV. This review will primarily focus on the issues related to the loading methods of therapeutic molecules into EVs for their clinical applications.
Physiological function of EVs
The ubiquitous presence of EVs in biological fluids and from almost all cell types in culture suggests a fundamental physiological function for these particles. While EVs may have different functions depending on their cellular origin, much attention was given to their role in tissue repair and regeneration. Tissue reparative potential of stem cells, mesenchymal stem cells (MSCs) in particular, have been extensively studied for last several decades (23,24). Recent studies revealed that most, if not all, of the tissue reparative activities are attributed to secretome of stem cells rather than cellular engraftment and integration (25,26). With its anti-inflammation/immunomodulatory, anti-fibrotic, anti-apoptotic, pro-angiogenetic activities and endogenous stem cell mobilization capacities, stem cell secretome became an ideal cell-free therapeutic strategy in a number of animal models, including myocardial infarct (27), liver diseases (28,29) and acute kidney injury (30). In particular, Lai et al. (31) showed that exosomes from conditioned medium of MSCs are the therapeutic entity for cardioprotection observed in their previous study (27). Their proposed roles in tissue homeostasis prompted their application in regenerative medicine. EVs from MSCs and other stem/progenitor cells demonstrated the tissue regenerative potential against acute and chronic kidney injury (32), heart muscle tissue after ischemic injuries of chronic myocardial infarction (33) and liver fibrosis (34). The diverse effects are from anti-fibrotic, inflammatory and proangiogenic activity of EVs. MSC-derived promoted functional recovery and neurovascular plasticity in animal model of ischemic stroke (35,36) via cytoprotective, anti-inflammatory, proangiogenic, anti-fibrotic and regenerative effects recapitulating the effects of EV donor cells. Because of their production ability, immune modulating capacity and clinical applicability, MSCs are the most preferred donor cell type (37). Yet, their innate angiogenic and tumor tissue homing characters, utilization of unmodified, but not chemical drug-loaded, EVs is not recommended in cancer therapy (38,39)
Unlike other drug delivery systems (DDS), EVs from stem/progenitor cells are considered to possess negligible immunogenicity as of their donor cells that lack MHC class II and co-stimulatory molecules (CD80, CD86 or CD40) (40). EVs from stem/progenitor cells are known to recapitulate immunosuppressive activity of their donor cells (41-43). In addition, EVs from cancer are the key contributor to tumor progression, metastasis and tumor-induced immune suppression (44). A recent study showed that allogeneic EVs from cardiosphere do not induce significant immune responses upon repeated subcutaneous injections [Mirotsou M, Blusztanj A, Tremmel I, et al. Repeated doses of cardiosphere-derived cell extracellular vesicles are hypo-immunogenic. J Extracell Vesicles, Proceedings of the Abstracts from the 4th International Meeting of ISEV (ISEV’15), 2015] as of the parental cells in a clinical study (45) suggesting that allogeneic EVs can be suitable for clinical applications.
On the contrary, EVs from professional antigen presenting cells, such as dendritic cells, B cells and macrophages are known to express functional immune modulating proteins including MHC-class I and/or MHC class-II (46-48) and EVs from these cells preferentially induce Th1-type (cell-mediated) immune response that directs T cells to attack abnormal cells (such as cancer cells) or cells infected with intracellular parasites (49). Yet, EVs from immature or regulatory DCs are known to exert immunosuppressive activities via inducing antigen-specific regulatory T cell activation in animal models of allograft tissue tolerance (50,51) or autoimmune diseases (52,53). Thus, care must be taken in selecting EVs from different cellular origins that is suitable for its intended use.
Although the identity of therapeutic factors in EVs are under intense debate, it is clear that RNA species (mRNA, miRNA and lncRNAs) in EVs are functionally transferred to recipient cells (14,54) and modulate the behavior of target cells. In addition to their tissue regenerative potential, researchers are exploring these particles as potential diagnostic tools (55,56) or delivery vehicles for therapeutic molecules (57,58).
EVs as drug delivery vehicles
Among the innovative drug delivery technology developed, EVs holds a great deal of promise for targeted drug delivery. While synthetic nanoparticles, liposomes and recombinant viral vectors have been exploited as therapeutic vehicles, even with extensive modification and formulation, their toxicity, bioavailability and target delivery were the key issues. For example, chemical modification (such as PEGylation or chitosan) of nanoparticles or liposomes can efficiently increase their systemic bioavailability while interfering their interaction with target cells thereby reducing their biodistribution in target tissues (59-61). Furthermore, these changes significantly increase the immunogenicity and induce immune response against the carriers thereby increasing their clearance upon subsequent injection (62,63). In this regard, nano-sized natural EVs represent an excellent alternative for drug delivery. As the composition of EV’s membrane is from donor cell (stem cells), these particles are non-immunogenic in nature allowing them to resist to fast clearance from circulation and thereby increasing the drug delivery efficiency to target tissues (64,65). As of their cellular counterparts, EVs are known to possess specific cell tropism or homing ability (66,67) by cell type specific proteins (with their surface ligand and adhesion molecules), one of the key requirements for targeted drug delivery.
Over 98% of potent drugs for central nervous system failed to exhibit meaningful activity in the brain and many show poor penetration of the blood brain barrier (BBB) (68). In this regard, EVs are the ideal DDS for BBB, as these particles are known to possess an ability to cross biological barriers and deliver proteins, RNAs, DNA and chemical drugs. Thus, EVs possess advantages of both synthetic drug carriers and cell-mediated therapeutics, while avoiding the inherent limitations associated with synthetic carriers and cellular therapeutics. In addition, EVs can be formulate to exhibit intended drug carrying activity through various approaches including biological, chemical and physical means. Encapsulation of drugs (chemicals, RNAs, DNA, proteins or lipids) into EVs can greatly increase their bioavailability by preserving their integrity and biological activity in vivo. Lipid membrane from donor cells are suited to avoid phagocytosis, degradation and modification in host circulation. In addition, these natural products of our body typically avoid entrapment in reticuloendothelial system (also known as mononuclear phagocytic system) and non-immunogenic in most, if not all, parameters. Hence, extensive studies are being explored this natural product for the delivery of drugs (therapeutic chemicals, nucleic acids and proteins).
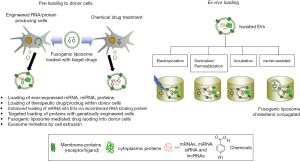
Various approaches can be utilized for loading of therapeutic agents into EVs. These include (I) loading drugs (of chemical, proteins or genetic materials) to purified EVs ex vivo, (II) pre-loading of drug or therapeutic factors to donor cells prior to EV purification (Figure 2).
Ex vivo drug loading to purified EVs
Ex vivo loading strategies mostly utilize passive packaging of therapeutic molecules, ranging from simple incubation to more sophisticated chemical and/or physical methods. Hydrophobic (i.e., lipophilic) molecules, such as anti-oxidants, anti-cancer drugs, lipophilic dyes, can be spontaneously packaged into EV under ambient conditions. Indeed, successful loading of curcumin (69), doxorubicin (70) and paclitaxel (71) into EVs were demonstrated. Extensive studies have been done in Curcumin, a natural polyphenol with strong anti-inflammatory property (57). Curcumin was incorporated into EVs by mixing to enhance the bioavailability and effectiveness of this hydrophobic chemical. Compared to standard liposomes composed of phosphatidylcholine and cholesterol, EVs exhibit higher loading efficiency and loading capacity to hydrophobic chemical drugs (72). The desired functional capacities of EVs isolated by sucrose gradient centrifugation were observed in suppression of macrophage activation in vitro and in lipopolysaccharides (LPS)-induced septic shock animal model in vivo. Of particular interest to the field of EVs as DDS is cancer therapy. The elegant study of Yang et al. (73) provided evidence that EVs possess the ability to deliver drugs across the BBB where exosomes isolated from brain tumor cell lines (U-87, PFSK-1, A-712 and bEND.3) loaded with rhodamine 123 and paclitaxel or doxorubicin could be detected in the brain of zebrafish embryos. Chemical drugs can also be loaded into EVs by electroporation (58). Studies demonstrated the enhanced efficacy with decreased adverse effects typically associated with chemotherapeutic drugs when compared to either EV-free drugs or drug-loaded liposomes (74,75). Yet, the efficiency of drug loading by these methods is less than expected, possibly due to the cargo capacity of purified EVs that carrying numerous cellular proteins and RNAs in it. For example, drug loading efficiency for paclitaxel and doxorubicin was 7.2% and 11.7%, respectively, determined by HPLC (73). In addition, the accurate measurement of EV-loaded drugs is not easy and their relative activity can only be referred to the protein contents in the complex.
EVs are the natural carriers of various nucleic acids including mRNA, miRNA and various noncoding RNAs (76,77) and thus represent ideal vehicles for nucleic acid transfer. Although siRNA is effective means for the regulation of genes of interests, their low stability and transducibility in circulation dictates the necessity of vehicles that can protect and deliver these therapeutic molecules to target cells and tissues. For ex vivo transfer of genetic materials to EVs, electroporation was firstly introduced in siRNA loading into exosomes derived from dendritic cells (58). Electrical field was applied to create pores in the membrane of EVs temporally, thereby allowing the movement of siRNA into the lumen of EVs. The delivery of and selective silencing of target genes by siRNA loaded EVs has been validated in a number of studies (78,79). Although loading efficiency of siRNA into EVs in these studies were up to 25% as determined by fluorescence spectroscopy of labeled siRNA (58,78), the actual loading efficiency in electroporation measured by Nanoparticle tracking analysis and confocal microscopy appears to be far less efficient than reported (80). Electroporation is also known to induce vesicular aggregation thereby affecting the integrity of the vesicles. While electroporation can be a suitable method for clinical setting as it can be easily controlled, several parameters including EV sources and concentrations, the cargo molecules (miRNA, mRNA, siRNA, lncRNA or plasmids) and the applying voltage with time for electroporation are difficult to standardize for optimal loading of the therapeutic cargo. The loading efficiency and capacity of exogenous DNA appears to be dependent on DNA size (linear DNA less than 1 kb is more efficient than large nucleic acids (81). In an effort to overcome this limitation, Wahlgren et al. (78) proposed a strategy to increase the incorporation of exogenous nucleic acids to EVs by utilizing pre-complexation of cationic liposome or micelle with siRNA and subsequent fusion with EVs that naturally carry a negative charge. Although two studies utilized chemical transfection reagents for siRNA loading into EVs, the efficiency could not be quantified and controlled (78,82) and making them not suitable for the clinical translation. To circumvent the utilization of harsh chemical or physical insults that may compromise the integrity of EVs, Didiot et al. (83) developed a robust and scalable methods for loading therapeutic RNA into EVs. The hydrophobically modified siRNA targeting Huntington RNA were efficiently loaded into EVs without altering the size and integrity of EVs. The silencing of Huntington mRNA by EVs was demonstrated in vitro using mouse primary cortical neurons as well as in vivo upon infusion into mouse striatum. Cholesterol-conjugation of siRNA is an efficient and reproducible method for siRNA loading into EVs (84). Sonication can be a suitable alternative for active loading of siRNA with minimal aggregation and degradation (85). With these approaches, the manufacturing processes for EVs loaded with exogenous nucleic acids can be scaled up for clinical uses with controllable loading efficiency.
Aside from nucleic acids, EVs are a natural carrier of proteins (15). Since proteins cannot penetrate EV membrane freely, loading of EVs with proteins is a most challenging as it utilizes tactics to destabilize lipid bilayers by means of sonication, permeabilization, fusogenic liposomes, polymeric carriers and other physical insults. Haney et al. (86) reported that ex vivo catalase loading into EVs using various methods for Parkinson’s disease therapy; the incubation at room temperature, permeabilization with saponin, freeze-thaw cycles, sonication, or extrusion. The study showed that a sonication and extrusion, or permeabilization with saponin resulted in stable EV reformation with high loading efficiency (18–26% measured by enzymatic activity), sustained release, preservation against proteases degradation and targeted delivery of this 240 kD protein in vitro and in vivo. Intranasal delivery of catalase-loaded EVs provided significant anti-inflammatory and neuroprotective effects with behavioral recovery in 6-OHDA treated mouse model of Parkinson’s disease demonstrating the ability of EVs to deliver therapeutic proteins across BBB for the treatment of various neurodegenerative disorders. However, this protein loading method based on mechanical dispersion of EVs as well as protein denaturation or destabilization may significantly limit its applicability in clinics for unstable proteins.
Pre-loading of drugs to donor cells
Therapeutic agents such as chemical drugs can be incorporated to EVs from host cells. Pascucci et al. (71) demonstrated that EVs isolated from chemical drug-treated MSCs exhibited anti-proliferative activity to cancer cells in vitro. In another study, Lv et al. (87) reported that EVs isolated from cancer cells treated with different chemical drugs, such as paclitaxel, carboplatin, etoposide and irinotecan, exhibited strong anti-proliferative activity to cancer cells as well as NK stimulatory activity in vitro. Although these studies elegantly demonstrated the successful drug loading of EV in vivo, the pitfall of this approach is an inability to control the loading efficiency. Jang et al. (74) developed an efficient method to generate large quantity of exosome-mimetic nanovesicles by breakdown of drug-loaded donor cells (monocytes/macrophages) that exhibit efficient antitumor activity in vivo. In the same study, the authors demonstrated that removal of membrane proteins by trypsinization eliminated the therapeutic activity of the engineered EVs in vitro as well as in vivo implying the essential role of membrane proteins in tissue targeting and/or information transfer. More recently, Lee et al. (88) developed a liposome-mediated MV engineering for anticancer drug-loading where synthetic fusogenic liposomes loaded with chemical drugs (hydrophobic sensitizers as a model drug) were efficiently incorporated into host cell membrane and subsequently loaded into EVs.
Since EVs are the natural carriers of genetic materials, such as mRNA, miRNA and various noncoding RNA in vivo, the most preferred strategy is that utilizing EVs from donor cells transfected with therapeutic genes. Several studies harnessed the mechanism for generating EVs carrying high level of miRNA expressing vectors (89,90). EVs from miR-146b transfected MSCs effectively inhibited the glioma growth in vitro as well as in rat brain (90). EVs from miR-210 transfected neural progenitor cells (NPCs) protected endothelial cells from angiotensin II-induced oxidative stress (91). Alternatively, suppression of a miR-9, the key miRNA conferring chemoresistance of glioblastoma, can be achieved by EVs from anti-miR-transfected MSCs (92). Targeting of therapeutic miRNA-loaded EVs can be greatly enhance by utilization of the strategy in growth factor receptor-growth factor ligand interaction. Ohno et al. (89) showed that an efficient loading of let-7a miRNA to EVs and in vivo cancer targeting strategy by utilizing EGFR-binding EGF or GE11 domain on the surface of EVs. Monitoring with in vivo imaging system (IVIS) revealed that the XenoLight DiR-labeled GE11-positive EV localized mainly in the tumor at 24 h after intravenous injection, while little signal was detected in native exosome indicating the successful tumor targeting. The finding that sumoylated form of ubiquitously expressed RNA-binding protein, heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1), directs the sorting of miRNA loading into exosome through recognition of short sequence motifs over-represented in miRNAs (EXOmotifs) (93) provide a tool for efficient loading of selected regulatory miRNAs into EVs.
Since proteins are the major components of EVs, loading of recombinant therapeutic proteins expressed by host cells can be an attractive mode of drug delivery. A number of model proteins, including ovalbumin, catalase, glial cell-line derived neurotropic factor (GDNF) was successfully loaded into EVs from gene-modified host cells (94,95). Targeting EVs to specific tissues can be achieved by surface protein of EV membrane. Grapp et al. (96) demonstrated that intraventricular injection of EV expressing folate receptor-α on its surface can cross the BBB and deliver therapeutics into brain parenchyma implying that utilization of these EV membrane proteins can greatly enhance the drug targeting to brain for treatment of neurodegenerative disease or malignancy. EVs from Epstein Barr virus-transformed or infected cells expressing gp350 protein can be used to transfer the contents to CD21 expressing B cells (97). Similarly, EVs from dendritic cells or exosome-mimetic nanovesicles from monocyte/macrophage expressing LFA-1 facilitate the interaction of EVs with activated T cells for delivering tumor antigens to induce tumor-specific immunity (98) or with tumor-associated endothelial cells for delivering chemotherapeutics to tumor cells (74), respectively. Studies have shown that tetraspanins, highly enriched in exosomes are known to participate in target cell binding via integrin binding (99,100). Thus, the delineation of tetraspanin web of EVs and the exploitation of these information to target selection will facilitate targeted drug delivery (101).
The genetic modification of donor cells can be used for targeting of EVs to designated tissues. Yang et al. (102) reported that systemic administration of EVs from dendritic cells engineered to express lysosomal-associated membrane protein 2 (LAMP2) that were electroporated with model siRNA against GAPDH or BACE1, a therapeutic target of Alzheimer’s disease resulted in significant suppression of GAPDH or BACE1 in wild type mice. Similar study to Ohno et al. (89), tumor targeting of doxorubicin-loaded EVs from immature dendritic cells was greatly facilitated by engineering the fusion protein composed of LAMP2b with αV integrin-specific iRGD peptide (CRGDKGPDC) with less cardiac toxicity (70). Targeting of LAMP2b fusion proteins on the surface of EVs can be further enhanced by engineered glycosylation that protects the fusion proteins during biogenesis and secretion (103). For efficient targeting of therapeutic proteins that are not typically secreted or transported to plasma membrane, a recombinant DNA technology can be utilized. For example, a fusion gene encoding the vesicular targeting protein C1C2 and the model protein, ovalbumin, is engineered for ovalbumin loading into EVs (104). Yim et al. (105) recently developed a highly sophisticated method for intracellular delivery of target proteins during exosome biogenesis. They generated a cell line transfected with genes for two recombinant proteins, one for cryptochrome 2 (CRY2)-conjugated to target protein and the other for tetraspanin protein CD9 conjugated with CRY-interacting basic-helix-loop-helix 1 (CIB1) protein module. Since the photoreceptor cryptochrome 2 (CRY2) of Arabidopsis thaliana can bind to CIB1 by blue light wavelength, CRY2-conjugated cargo proteins can be actively and transiently docked into newly generated exosomes via CIB1 domain on CD9-CIB1 fusion proteins. Removal of blue light leads to detachment of target protein from CD9-CIB1 fusion proteins into the luminal space of exosomes. The potential of this strategy of protein delivery by exosome was further validated by transfer of Cre recombinase as a model protein in vitro as well as in vivo.
EVs can be engineered to deliver therapeutic mRNA/protein combination for cancer treatment. By transfecting donor cells (HEK-293T) with a vector construct consisting of cytosine deaminase (CD) fused to uracil phosphoribosyl transferase (UPRT), Mizrak et al. (106). generated MVs enriched with the suicide gene mRNA and protein. These studies validated that EVs may function as safe, efficient drug delivery vehicles to target tissues.
Therapeutic efficacy and toxicity of EVs are greatly influenced by their biodistribution (65). Studies on the various administration routes for efficient delivery of this therapeutic cargo to the lesions generated encouraging outcome (64,107). Of these, intravenous route is the most preferred route of administration. Like other DDS, systemic administration of EVs resulted in preferential accumulation in liver, kidney and spleen with rapid elimination in circulation. Multimodal imaging of systemically administered luciferase-loaded EVs in vivo revealed that the half-life of EVs were less than 30 min in most tissue and they were mostly cleared from the animals by 6 h (108). Similarly, a pharmacokinetic analysis revealed that the half-life of EV loaded with luciferase-lactadherin fusion protein from murine melanoma cells EV in the circulation is approximately 2 min and weakly detectable after 4 h indicating rapid clearance in vivo (109). Accumulation of EVs in the lung, liver, bone marrow and spleen was observed by sequential in vivo imaging suggesting that the distribution reflect the organotropism of murine melanoma during metastasis. These results are in sharp contrast to previous studies demonstrating that EVs can be detected in the liver and/or spleen, but not in circulation, at 24 h after systemic administration (89,110). The observed differences in biodistribution, tissue targeting efficiency and retention time in tissues may be attributed to the producing cells, vesicle sizes and the methods of EV preparation and thus warrant additional studies on the selection of optimal protocol for EV isolation, route of administration, formulation to increase their bioavailability and tissue targeting ability.
Conclusions
EVs clearly play key roles in normal physiology and diseases pathology. The use of drug-loaded EVs represent a next generation DDS with ability to transverse complex biological barriers such as BBB, while avoiding or overcoming a number of safety concerns related to drugs or vehicles, such as cytotoxicity, short biodistribution and low efficiency of targeted delivery. Chemical drugs and biological molecules (RNA, DNA and proteins) with low stability in circulation and/or low transducibility to target cells can be efficiently transferred to cytoplasm of target cells without undergoing endosomal and lysosomal degradation by this natural DDS. In addition, their composition and target specificity can be further tailored by engineering the producing cells or in vitro drug loading in accordance to target diseases or disorders; i.e., cancer therapy or tissue regeneration. A number of studies demonstrated that EVs may function as safe, efficient drug delivery vehicles to target tissues. Promising results of this cell-free therapeutics were obtained from a number of relevant animal models for human diseases and clinical translation of EVs has already initiated in cancer therapy and organ transplantation and their safety was validated.
However, the study of EVs is still in its early stages and a number of challenges remain to be addressed for their successful clinical translation. As shown by EVs from dendritic cells at different stages of maturation and MSCs, the heterogeneity in composition with functional activities of EVs is one of the key issues for their pharmaceutical acceptability. The choice of donor cells, culture conditions, refinement of methods for targeting and loading of therapeutic molecules must be explored in accordance to diseases. For clinical considerations of EVs, standardization of protocol for scalable production, isolation, storage and establishment of the criteria for quality control need to be established. Further study is required to better characterize the EVs and to delineate the underlying molecular mechanism responsible for their therapeutic effects and ultimately to exploit their full potential in the clinic.
Acknowledgements
Funding: This work was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIP), No. NRF-2017M3A9B4042583.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Trams EG, Lauter CJ, Salem N, et al. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta 1981;645:63-70. [Crossref] [PubMed]
- Caby MP, Lankar D, Vincendeau-Scherrer C, et al. Exosomal-like vesicles are present in human blood plasma. Int Immunol 2005;17:879-87. [Crossref] [PubMed]
- Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 2004;101:13368-73. [Crossref] [PubMed]
- Vella LJ, Sharples RA, Lawson VA, et al. Packaging of prions into exosomes is associated with a novel pathway of PrP processing. J Pathol 2007;211:582-90. [Crossref] [PubMed]
- Admyre C, Johansson SM, Qazi KR, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol 2007;179:1969-78. [Crossref] [PubMed]
- Ogawa Y, Kanai-Azuma M, Akimoto Y, et al. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull 2008;31:1059-62. [Crossref] [PubMed]
- Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med 1996;183:1161-72. [Crossref] [PubMed]
- van der Pol E, Böing AN, Harrison P, et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 2012;64:676-705. [Crossref] [PubMed]
- Denzer K, Kleijmeer MJ, Heijnen HF, et al. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 2000;113:3365-74. [PubMed]
- Escola JM, Kleijmeer MJ, Stoorvogel W, et al. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J Biol Chem 1998;273:20121-7. [Crossref] [PubMed]
- Théry C, Regnault A, Garin J, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol 1999;147:599-610. [Crossref] [PubMed]
- Conde-Vancells J, Rodriguez-Suarez E, Embade N, et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res 2008;7:5157-66. [Crossref] [PubMed]
- Llorente A, Skotland T, Sylvänne T, et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim Biophys Acta 2013;1831:1302-9. [Crossref] [PubMed]
- Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 2007;9:654-9. [Crossref] [PubMed]
- Kim HS, Choi DY, Yun SJ, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res 2012;11:839-49. [Crossref] [PubMed]
- Hugel B, Martínez MC, Kunzelmann C, et al. Membrane microparticles: two sides of the coin. Physiology 2005;20:22-7. [Crossref] [PubMed]
- Gan X, Gould SJ. Identification of an inhibitory budding signal that blocks the release of HIV particles and exosome/microvesicle proteins. Mol Biol Cell 2011;22:817-30. [Crossref] [PubMed]
- Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 2008;10:1470-6. [Crossref] [PubMed]
- Momen-Heravi F, Balaj L, Alian S, et al. Current methods for the isolation of extracellular Vesicles Biol Chem 2013;394:1253-62. [Crossref] [PubMed]
- Mathivanan S, Simpson RJ. ExoCarta: a compendium of exosomal proteins and RNA. Proteomics 2009;9:4997-5000. [Crossref] [PubMed]
- Xu R, Greening DW, Zhu HJ, et al. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest 2016;126:1152-62. [Crossref] [PubMed]
- Li P, Kaslan M, Lee SH, et al. Progress in exosome isolation techniques. Theranostics 2017;7:789-804. [Crossref] [PubMed]
- Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science 2000;287:1442-6. [Crossref] [PubMed]
- Kassem M. Stem cells: potential therapy for age-related diseases. Ann NY Acad Sci 2006;1067:436-42. [Crossref] [PubMed]
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076-84. [Crossref] [PubMed]
- Shen CY, Li L, Feng T, et al. Dental pulp stem cells derived conditioned medium promotes angiogenesis in hindlimb ischemia. Tissue Eng Reg Med 2015;12:59-68. [Crossref]
- Timmers L, Lim SK, Arslan F, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 2007;1:129-37. [Crossref] [PubMed]
- van Poll D, Parekkadan B, Cho CH, et al. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008;47:1634-43. [Crossref] [PubMed]
- Woo DH, Kim SK, Lim HJ, et al. Direct and indirect contribution of human embryonic stem cell-derived hepatocyte-like cells to liver repair in mice. Gastroenterology 2012;142:602-11. [Crossref] [PubMed]
- Zarjou A, Kim J, Traylor AM, et al. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol Renal Physiol 2011;300:F254-62. [Crossref] [PubMed]
- Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010;4:214-22. [Crossref] [PubMed]
- Gatti S, Bruno S, Deregibus MC, et al. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol Dial Transplant 2011;26:1474-83. [Crossref]
- Yamaguchi T, Izumi Y, Nakamura Y, et al. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol 2015;178:239-46. [Crossref] [PubMed]
- Li T, Yan Y, Wang B, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev 2013;22:845-54. [Crossref] [PubMed]
- Xin H, Li Y, Cui Y, et al. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab 2013;33:1711-5. [Crossref] [PubMed]
- Lee JY, Kim E, Choi SM, et al. Microvesicles from brain-extract-treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci Rep 2016;6:33038. [Crossref] [PubMed]
- Yeo RW, Lai RC, Zhang B, et al. Mesenchymal stem cell: An efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev 2013;65:336-41. [Crossref] [PubMed]
- Zhu W, Huang L, Li Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett 2012;315:28-37. [Crossref] [PubMed]
- Bruno S, Collino F, Deregibus MC, et al. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev 2013;22:758-71. [Crossref] [PubMed]
- Le Blanc K, Tammik C, Rosendahl K, et al. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 2003;31:890-6. [Crossref] [PubMed]
- Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007;110:2440-8. [Crossref] [PubMed]
- Del Fattore A, Luciano R, Pascucci L, et al. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Cell Transplant 2015;24:2615-27. [Crossref] [PubMed]
- Burrello J, Monticone S, Gai C, et al. Stem cell-derived extracellular vesicles and immune-modulation. Front Cell Dev Biol 2016;4:83. [Crossref] [PubMed]
- Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest 2016;126:1216-23. [Crossref] [PubMed]
- Barile L, Moccetti T, Marba’n E, et al. Roles of exosomes in cardioprotection. Eur Heart J 2017;38:1372-9. [PubMed]
- Lynch S, Santos SG, Campbell EC, et al. Novel MHC class I structures on exosomes. J Immunol 2009;183:1884-91. [Crossref] [PubMed]
- Chen W, Wang J, Shao C, et al. Efficient induction of antitumor T cell immunity by exosomes derived from heat-shocked lymphoma cells. Eur J Immunol 2006;36:1598-607. [Crossref] [PubMed]
- Segura E, Amigorena S, Théry C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis 2005;35:89-93. [Crossref] [PubMed]
- Viaud S, Théry C, Ploix S, et al. Dendritic cell-derived exosomes for cancer immunotherapy: what's next? Cancer Res 2010;70:1281-5. [Crossref] [PubMed]
- Li X, Li JJ, Yang JY, et al. Tolerance induction by exosomes from immature dendritic cells and rapamycin in a mouse cardiac allograft model. PLoS One 2012;7:e44045. [Crossref] [PubMed]
- Ma B, Yang JY, Song WJ, et al. Combining exosomes derived from immature DCs with donor antigen-specific Treg cells induces tolerance in a rat liver allograft model. Sci Rep 2016;6:32971. [Crossref] [PubMed]
- Bianco NR, Kim SH, Morelli AE, et al. Modulation of the immune response using dendritic cell-derived exosomes. Methods Mol Biol 2007;380:443-55. [Crossref] [PubMed]
- Yang X, Meng S, Jiang H, et al. Exosomes derived from interleukin-10-treated dendritic cells can inhibit trinitrobenzene sulfonic acid-induced rat colitis. Scand J Gastroenterol 2010;45:1168-77. [Crossref] [PubMed]
- Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther 2015;6:127. [Crossref] [PubMed]
- Müller G. Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. Diabetes Metab Syndr Obes 2012;5:247-82. [Crossref] [PubMed]
- Sinning JM, Jansen F, Hammerstingl C, et al. Circulating Microparticles Decrease After Cardiac Stress in Patients With Significant Coronary Artery Stenosis. Clin Cardiol 2016;39:570-7. [Crossref] [PubMed]
- Sun D, Zhuang X, Xiang X, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 2010;18:1606-14. [Crossref] [PubMed]
- Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011;29:341-5. [Crossref] [PubMed]
- Suk JS, Xu Q, Kim N, et al. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev 2016;99:28-51. [Crossref] [PubMed]
- Kraft JC, Freeling JP, Wang Z, et al. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J Pharm Sci 2014;103:29-52. [Crossref] [PubMed]
- Salehi M, Naseri-Nosar M, Azami M, et al. Comparative study of poly(L-lactic acid) scaffolds coated with chitosan nanoparticles prepared via ultrasonication and ionic gelation techniques. Tissue Eng Reg Med 2016;13:498-506. [Crossref]
- Dams ET, Laverman P, Oyen WJ, et al. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther 2000;292:1071-9. [PubMed]
- Ishida T, Kashima S, Kiwada H. The contribution of phagocytic activity of liver macrophages to the accelerated blood clearance (ABC) phenomenon of PEGylated liposomes in rats. J Control Release 2008;126:162-5. [Crossref] [PubMed]
- Johnsen KB, Gudbergsson JM, Skov MN, et al. A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta 2014;1846:75-87. [PubMed]
- Wiklander OP, Nordin JZ, O'Loughlin A, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles 2015;4:26316. [Crossref] [PubMed]
- Lai RC, Yeo RW, Tan KH, et al. Exosomes for drug delivery - a novel application for the mesenchymal stem cell. Biotechnol Adv 2013;31:543-51. [Crossref] [PubMed]
- Kooijmans SA, Schiffelers RM, Zarovni N, et al. Modulation of tissue tropism and biological activity of exosomes and other extracellular vesicles: New nanotools for cancer treatment. Pharmacol Res 2016;111:487-500. [Crossref] [PubMed]
- Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx 2005;2:3-14. [Crossref] [PubMed]
- Zhuang X, Xiang X, Grizzle W, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated antiinflammatory drugs from the nasal region to the brain. Mol Ther 2011;19:1769-79. [Crossref] [PubMed]
- Tian Y, Li S, Song J, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014;35:2383-90. [Crossref] [PubMed]
- Pascucci L, Coccè V, Bonomi A, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release 2014;192:262-70. [Crossref] [PubMed]
- Fuhrmann G, Serio A, Mazo M, et al. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release 2015;205:35-44. [Crossref] [PubMed]
- Yang T, Martin P, Fogarty B, et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm Res 2015;32:2003-14. [Crossref] [PubMed]
- Jang SC, Kim OY, Yoon CM, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano 2013;7:7698-710. [Crossref] [PubMed]
- Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancermolecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol 2013;65:157-70. [Crossref] [PubMed]
- Wang K, Zhang S, Weber J, et al. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res 2010;38:7248-59. [Crossref] [PubMed]
- Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet 2012;21:R125-34. [Crossref] [PubMed]
- Wahlgren J, De L, Karlson T, Brisslert M, et al. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res 2012;40:e130. [Crossref] [PubMed]
- Pan Q, Ramakrishnaiah V, Henry S, et al. Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference (RNAi). Gut 2012;61:1330-9. [Crossref] [PubMed]
- Kooijmans SA, Stremersch S, Braeckmans K, et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release 2013;172:229-38. [Crossref] [PubMed]
- Lamichhane TN, Raiker RS, Jay SM. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol Pharm 2015;12:3650-7. [Crossref] [PubMed]
- Shtam TA, Kovalev RA, Varfolomeeva EY, et al. Exosomes are natural carriers of exogenous siRNA to human cells in vitro Cell Commun Signal 2013;11:88. [Crossref] [PubMed]
- Didiot MC, Hall LM, Coles AH, et al. Exosome-mediated delivery of hydrophobically modified siRNA for Huntingtin mRNA silencing. Mol Ther 2016;24:1836-47. [Crossref] [PubMed]
- Stremersch S, Vandenbroucke RE, Van Wonterghem E, et al. Comparing exosome-like vesicles with liposomes for the functional cellular delivery of small RNAs. J Control Release 2016;232:51-61. [Crossref] [PubMed]
- Lamichhane TN, Jeyaram A, Patel DB, et al. Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell Mol Bioeng 2016;9:315-24. [Crossref] [PubMed]
- Haney MJ, Klyachko NL, Zhao Y, et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release 2015;207:18-30. [Crossref] [PubMed]
- Lv LH, Wan YL, Lin Y, et al. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem 2012;287:15874-85. [Crossref] [PubMed]
- Lee J, Kim J, Jeong M, et al. Liposome-based engineering of cells to package hydrophobic compounds in membrane vesicles for tumor penetration. Nano Lett 2015;15:2938-44. [Crossref] [PubMed]
- Ohno S, Takanashi M, Sudo K, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 2013;21:185-91. [Crossref] [PubMed]
- Katakowski M, Buller B, Zheng X, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett 2013;335:201-4. [Crossref] [PubMed]
- Liu H, Wang J, Chen Y, et al. NPC-EXs alleviate endothelial oxidative stress and dysfunction through the miR-210 downstream Nox2 and VEGFR2 pathways. Oxid Med Cell Longev 2017;2017:9397631. [PubMed]
- Munoz JL, Bliss SA, Greco SJ, et al. Delivery of functional anti-miR-9 by mesenchymal stem cell-derived exosomes to glioblastoma multiforme cells conferred chemosensitivity. Mol Ther Nucleic Acids 2013;2:e126. [Crossref] [PubMed]
- Villarroya-Beltri C, Gutiérrez-Vázquez C, Sánchez-Cabo F, et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 2013;4:2980. [Crossref] [PubMed]
- Marcus ME, Leonard JN. FedExosomes: engineering therapeutic biological nanoparticles that truly deliver. Pharmaceuticals (Basel) 2013;6:659-80. [Crossref] [PubMed]
- Haney MJ, Zhao Y, Harrison EB, et al. Specific transfection of inflamed brain by macrophages: a new therapeutic strategy for neurodegenerative diseases. PLoS One 2013;8:e61852. [Crossref] [PubMed]
- Grapp M, Wrede A, Schweizer M, et al. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun 2013;4:2123. [Crossref] [PubMed]
- Vallhov H, Gutzeit C, Johansson SM, et al. Exosomes containing glycoprotein 350 released by EBV-transformed B cells selectively target B cells through CD21 and block EBV infection in vitro. J Immunol 2011;186:73-82. [Crossref] [PubMed]
- Nolte-'t Hoen EN, Buschow SI, Anderton SM, et al. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood 2009;113:1977-81. [Crossref] [PubMed]
- Gesierich S, Berezovkiy I, Ryschich E, et al. Systemic angiogenesis induction by the tetraspanin D6.1A. Cancer Res 2006;66:7083-94. [Crossref] [PubMed]
- Nazarenko I, Rana S, Baumann A, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res 2010;70:1668-78. [Crossref] [PubMed]
- Rana S, Yue S, Stadel D, et al. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol 2012;44:1574-84. [Crossref] [PubMed]
- Yang J, Zhang X, Chen X, et al. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol Ther Nucleic Acids 2017;7:278-87. [Crossref] [PubMed]
- Hung ME, Leonard JN. Stabilization of exosome-targeting peptides via engineered glycosylation. J Biol Chem 2015;290:8166-72. [Crossref] [PubMed]
- Zeelenberg IS, Ostrowski M, Krumeich S, et al. Targeting tumor antigens to secreted membrane vesicles in vivo induces efficient antitumor immune responses. Cancer Res 2008;68:1228-35. [Crossref] [PubMed]
- Yim N, Ryu SW, Choi K, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. . Nat Commun 2016;7:12277. [Crossref] [PubMed]
- Mizrak A, Bolukbasi MF, Ozdener GB, et al. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther 2013;21:101-8. [Crossref] [PubMed]
- Lener T, Gimona M, Aigner L, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles 2015;4:30087. [Crossref] [PubMed]
- Lai CP, Mardini O, Ericsson M, et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 2014;8:483-94. [Crossref] [PubMed]
- Takahashi Y, Nishikawa M, Shinotsuka H, et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol 2013;165:77-84. [Crossref] [PubMed]
- Peinado H, Alečković M, Lavotshkin S, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012;18:883-91. [Crossref] [PubMed]
Cite this article as: Kim SM, Kim HS. Engineering of extracellular vesicles as drug delivery vehicles. Stem Cell Investig 2017;4:74.


