CALR-positive myeloproliferative disorder in a patient with Ph-positive chronic myeloid leukemia in durable treatment-free remission: a case report
Introduction
Among the classic myeloproliferative neoplasia (MPN), chronic myeloid leukemia (CML) is characterized by a pathognomonic Philadelphia (Ph)-chromosome, corresponding to translocation t(9;22) and resulting in the BCR-ABL1 oncogene. The classical studies of Fialkow et al., based on the analysis of X-linked glucose 6-phosphate dehydrogenase (G6PDH) polymorphisms in hemopoietic cells of CML female patients carrying a GDPDH heterozygosity (1), have shown that the Ph-chromosome arises in a multipotent stem cell and can be observed in all hemopoietic lineages, including B lymphocytes. This finding has been confirmed in further studies, using high sensitivity techniques such as fluorescent-in situ hybridization on sorted cells (2,3). The possibility of a multistep pathogenesis for CML was suggested by the demonstration of an identical G6PDH pattern and multiple chromosomal aberrations in Ph-negative B-lymphoid cell lines derived from CML patients. This observation implies that at least two steps are required to develop CML phenotype, one causing abnormal proliferation at pluripotent stem cell level, and one inducing Ph chromosome in the descendants of these progenitors (4). On this basis, the so called “Fialkow hypothesis” suggested that a clonal hemopoiesis with genetic instability may evolve into CML by acquisition of the Ph-chromosome.
Similar to CML, Ph-negative MPN also arise in a multipotent hemopoietic stem cell (5). In 2005, the discovery that Janus kinase 2 (JAK2) gene is involved in polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF) has refined current diagnostic criteria of MPN (6). In 2013, calreticulin (CALR) gene was discovered to be involved in JAK2 negative myeloproliferative disorders (7,8) and the mutation of these two genes, together with mutations of thrombopoietin receptor gene [myeloproliferative leukemia (MPL)] (9), were found to be mutually exclusive (10,11).
The 2016 revision of the WHO myeloid neoplasm classification takes into account the discovery of these novel molecular findings, but it does not address the classification of MPN cases that present more than one genetic abnormality. However, in clinical practice the coexistence of different abnormalities has been previously described: in particular, the association of JAK2 positive MPN and Ph-positive CML is considered rare, but more than 25 cases were reported in literature (12). Sporadic, rare cases of coexistence of CALR-mutated MPN and Ph-positive CML have been recently described too. Bonzheim et al. reported one case of BCR-ABL1 translocation arising in a patient with previous diagnosis of thrombocytosis, identified as CALR-mutated ET (13); it was hypothesized in a similar case by Cabagnols et al. that a common clone harbored both genetic alterations, CALR mutation being pre-acquired and BCR-ABL1 translocation being a secondary event (14). Two other cases of co-existence of chronic phase CML and CALR mutation were described, with a CALR-positive PMF diagnosed 7 to 24 months after the start of tyrosine kinase inhibitors (TKI) therapy for CML; bone marrow (BM) examination revealed moderate fibrosis in one case, and prominent collagen fibrosis in the other case (15,16). In a recent case by Seghatoleslami, del52CALR mutation with a high allele burden was found to be present at diagnosis of a blast phase CML, associated with the uncommon p190 Bcr-Abl1 transcript positivity (17).
Here we report a case of coexistence of BCR-ABL1 fusion gene and CALR mutation, diagnosed due to persistent thrombocytosis while the patient was in treatment-free, complete molecular remission (CMR) from CML.
Case presentation
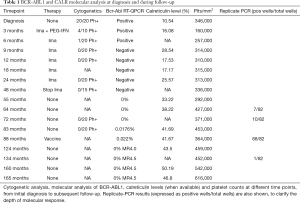
A 61-year-old woman was diagnosed with Ph+ CML, low Sokal risk, in May 2001. She was initially treated with hydroxyurea until August, when she was enrolled in a GIMEMA protocol (CML 011) (18): she was then administered imatinib 400 mg per day in association with PEG-interferon (IFN) 50 mcg/week front line, which was stopped for skin toxicity after 4 months. She achieved complete cytogenetic remission (CCyR) and CMR, defined as undetectable Bcr-Abl1 transcript at qualitative real time PCR (RT-PCR), within 9 months of treatment, in May 2002. CMR was stable for 41 months and imatinib was discontinued in October 2010 due to gastric intolerance. The patient stayed off treatment in confirmed CMR, with undetectable Bcr-Abl1 transcript on both BM and peripheral blood (PB), in multiple serial samples, for 28 months.
In February 2008, a RT quantitative PCR (RT-qPCR) analysis on PB disclosed a Bcr-Abl1 /Abl1 ratio of 0.0208%, consistent with a MMR.
To determine the depth of Bcr-Abl1 positivity reached we also performed Replicate RT-qPCR, a novel, high-sensitivity PCR. The assay was carried out using custom-plates provided by Life Technologies, designed to include: 82 wells for Bcr-Abl1 detection and quantification, 2 wells for Abl1 (used as control gene), 2 wells for no template control for Bcr-Abl1, 2 wells for no template control for Abl1, 4 wells for the unique plasmid DNA to obtain the standard curve for Bcr-Abl1, 4 wells for the unique plasmid DNA to obtain the standard curve for Abl1 (19,20). Nine µg of total RNA of each sample were retro-transcribed according to the EAC protocol. Each well of the plate contained a cDNA amount corresponding to 100 ng of the initial RNA. Final results were expressed as qualitative (percentage of positive wells/82 wells) and quantitative (mean Bcr-Abl1 copies/mean Abl1 copies %) values, reaching a sensitivity equal to MR5.5/MR 6. Replicate-PCR was also performed on 14 healthy controls to establish a positivity threshold for the test, obtained as mean +2 standard deviation of the number of positive wells in the controls (2.7/82 wells).
Thanks to this high sensitivity, test results showed that Bcr-Abl1 was detectable before a positive analysis with standard method was observed.
The patient refused to start imatinib again, and control samples performed every 3 to 6 months thereafter maintained very low Bcr-Abl1 levels, ranging between 0% and 0.022%, without ever losing CCyR or major molecular remission (MMR). In March 2010, she was enrolled in a peptide vaccination protocol (CML VAX) (21), that she continued for a total of 17 doses until April 2014, showing no side effects; in this phase, molecular monitoring showed negativity of Bcr-Abl1 transcript since November 2011 (after 10 doses of vaccine). After starting the CML VAX protocol the number of positive wells decreased. The patient remained in CMR, with undetectable transcript until the latest follow-up, performed in October 2016, 181 months since the initial diagnosis and almost 12 years since the discontinuation of TKI therapy, marking one of the longest treatment-free remissions (TFR) from CML obtained so far (22,23). It is conceivable that in this particular case, in addition to the effect of TKI, a major role was exerted by immunologic response, enhanced by CML VAX (24).
Due to a rising platelet count in subsequent follow-up blood drawn (maximum reached was 616,000/mm3) while CML was well controlled, in order to exclude the coexistence of CML and a Ph-negative myeloproliferative syndrome, in February 2015, we performed a panel of myeloproliferative molecular mutations on a BM aspiration sample: the patient had no JAK2 V617F mutation, but CALR mutation was present, allelic burden being 46.8%. A 52 bp deletion in exon 9 of CALR (type1 CALR mutation) was in fact identified using capillary fragment analysis and the CALR mutation was confirmed using bi-directional DNA sequencing. Biopsy showed a myeloproliferative BM that was interpreted as ET or possibly PMF at a pre-fibrotic stage. At that time, she was off therapy for CML, steadily in CMR.
We then retrospectively repeated CALR quantitative analysis on previous blood samples, to determine when the CALR clone had emerged. We found that CALR mutation had been present (10.5%) since the moment of the CML diagnosis, in 2001; its levels, as well as platelet count, slowly increased through time, while the patient first gained CCyR from CML, 9 months after the initial diagnosis, and BCR-ABL1 transcript decreased until undetectability in May 2006 (Table 1).
Full table
Discussion
Our patient retrospectively presented a CALR mutation since the initial diagnosis of CML, but CALR allelic burden rose and became phenotypically relevant, producing thrombocytosis, after CMR from CML and when TKI-independence was obtained. Therefore, it is possible that in this particular case, unlike in the case previously described by Cabagnos et al., two independent clones had carried the two molecular alterations: BCR-ABL1-positive clone was dominant at the beginning, but responded rapidly to TKI therapy and soon became undetectable. Meanwhile, CALR-positive clone could have gained selective advantage from the inhibition of the Ph-positive clone exerted first by imatinib, later by CML VAX immunotherapy, and consequently started to grow, producing thrombocytosis that prompted a screening for Ph-negative diseases.
As stated by Fialkow in 1981 for CML, chronic MPN probably arise via a multistep pathogenesis, including an early step in hematopoietic stem cells (HSC), able to determine the initial clonal proliferation and a following subclonal evolution because of chromosomal instability (4). Thus, it is also possible that in our case an earlier event occurred in HSC, leading to the subsequent development of two different mutated subclones, respectively BCR-ABL1-positive and CALR-positive.
As both CML and Ph-negative MPN are disorders of the HSCs, the coexistence in a number of patients of multiple genetic lesions, partially sensitive to TKIs treatment, may be a clue to evaluate the clonal evolution of the leukemic stem cell population.
Conclusions
We reported the case of a CML patient that reached CMR and maintained TKI TFR for almost 12 years, associated with a CALR-mutated ET; the association of Ph-positive CML in persistent CMR and of CALR-positive ET has not been described before. However, few other cases reporting the coexistence of BCR-ABL1 and other Ph-negative MPN markers (CALR or JAK2 mutations) have been described: in these cases, the pattern of clonal evolution, that is, the timing of the different genetic abnormalities and the “stemness” level of the cell involved in the first alteration, was highly heterogeneous. Investigating the coexistence of BCR-ABL1 and other Ph-negative MPN markers at different time-points may help interpretation of the various clinical and pathogenetic features of myeloproliferative diseases. Although current guidelines do not recommend to perform a molecular screening for CALR mutations when a Ph-positive CML has been diagnosed, molecular analysis for JAK2 and CALR mutations may be indicated in selected cases.
Acknowledgements
This work was supported by fellowship from Associazione Italiana Leucemie to F Daraio and F Carnuccio.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Fialkow PJ, Denman AM, Jacobson RJ, et al. Chronic myelocytic leukemia. Origin of some lymphocytes from leukemic stem cells. J Clin Invest 1978;62:815-23. [Crossref] [PubMed]
- Takahashi N, Miura I, Saitoh K, et al. Lineage involvement of stem cells bearing the philadelphia chromosome in chronic myeloid leukemia in the chronic phase as shown by a combination of fluorescence-activated cell sorting and fluorescence in situ hybridization. Blood 1998;92:4758-63. [PubMed]
- Holyoake TL, Jiang X, Drummond MW, et al. Elucidating critical mechanisms of deregulated stem cell turnover in the chronic phase of chronic myeloid leukemia. Leukemia 2002;16:549-58. [Crossref] [PubMed]
- Fialkow PJ, Martin PJ, Najfeld V, et al. Evidence for a multistep pathogenesis of chronic myelogenous leukemia. Blood 1981;58:158-63. [PubMed]
- Raskind WH, Jacobson R, Murphy S, et al. Evidence for the involvement of B lymphoid cells in polycythemia vera and essential thrombocythemia. J Clin Invest 1985;75:1388-90. [Crossref] [PubMed]
- James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144-8. [Crossref] [PubMed]
- Nangalia J, Massie CE, Baxter EJ, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369:2391-405. [Crossref] [PubMed]
- Klampfl T, Gisslinger H, Harutyunyan AS, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369:2379-90. [Crossref] [PubMed]
- Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006;3:e270. [Crossref] [PubMed]
- Tefferi A, Pardanani A. Myeloproliferative Neoplasms: A Contemporary Review. JAMA Oncol 2015;1:97-105. [Crossref] [PubMed]
- Wojtaszewska M, Iwola M, Lewandowski K. Frequency and molecular characteristics of calreticulin gene (CALR) mutations in patients with JAK2 -negative myeloproliferative neoplasms. Acta Haematol 2015;133:193-8. [Crossref] [PubMed]
- Hummel JM, Kletecka MC, Sanks JK, et al. Concomitant BCR-ABL1 translocation and JAK2(V617F) mutation in three patients with myeloproliferative neoplasms. Diagn Mol Pathol 2012;21:176-83. [Crossref] [PubMed]
- Bonzheim I, Mankel B, Klapthor P, et al. CALR-mutated essential thrombocythemia evolving to chronic myeloid leukemia with coexistent CALR mutation and BCR-ABL translocation. Blood 2015;125:2309-11. [Crossref] [PubMed]
- Cabagnols X, Cayuela JM, Vainchenker W. A CALR mutation preceding BCR-ABL1 in an atypical myeloproliferative neoplasm. N Engl J Med 2015;372:688-90. [Crossref] [PubMed]
- Loghavi S, Pemmaraju N, Kanagal-Shamanna R, et al. Insights from response to tyrosine kinase inhibitor therapy in a rare myeloproliferative neoplasm with CALR mutation and BCR-ABL1. Blood 2015;125:3360-3. [Crossref] [PubMed]
- Diamond JM, de Almeida AM, Belo HJ, et al. CALR-mutated primary myelofibrosis evolving to chronic myeloid leukemia with both CALR mutation and BCR-ABL1 fusion gene. Ann Hematol 2016;95:2101-4. [Crossref] [PubMed]
- Seghatoleslami M, Ketabchi N, Ordo A, et al. Coexistence of p190 BCR/ABL Transcript and CALR 52-bp Deletion in Chronic Myeloid Leukemia Blast Crisis: A Case Report. Mediterr J Hematol Infect Dis 2016;8:e2016002. [Crossref] [PubMed]
- Palandri F, Iacobucci I, Castagnetti F, et al. Front-line treatment of Philadelphia positive chronic myeloid leukemia with imatinib and interferon-alpha: 5-year outcome. Haematologica 2008;93:770-4. [Crossref] [PubMed]
- Goh HG, Lin M, Fukushima T, et al. Sensitive quantitation of minimal residual disease in chronic myeloid leukemia using nanofluidic digital polymerase chain reaction assay. Leuk Lymphoma 2011;52:896-904. [Crossref] [PubMed]
- Koren-Michowitz M, Shimoni A, Daraio F, et al. Sensitive Replicate Real-Time Quantitative PCR of BCR-ABL Shows Deep Molecular Responses in Long-Term Post-Allogeneic Stem Cell Transplantation Chronic Myeloid Leukemia Patients. Biol Blood Marrow Transplant 2015;21:1852-5. [Crossref] [PubMed]
- Bocchia M, Defina M, Aprile L, et al. Complete molecular response in CML after p210 BCR-ABL1-derived peptide vaccination. Nat Rev Clin Oncol 2010;7:600-3. [Crossref] [PubMed]
- Mahon FX, Rea D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol 2010;11:1029-35. [Crossref] [PubMed]
- Etienne G, Guilhot J, Rea D, et al. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients With Chronic Myeloid Leukemia. J Clin Oncol 2017;35:298-305. [Crossref] [PubMed]
- Ilander M, Hekim C, Mustjoki S. Immunology and immunotherapy of chronic myeloid leukemia. Curr Hematol Malig Rep 2014;9:17-23. [Crossref] [PubMed]
Cite this article as: Dogliotti I, Fava C, Serra A, Gottardi E, Daraio F, Carnuccio F, Giugliano E, Bocchia M, Saglio G, Rege-Cambrin G. CALR-positive myeloproliferative disorder in a patient with Ph-positive chronic myeloid leukemia in durable treatment-free remission: a case report. Stem Cell Investig 2017;4:57.


