The mammary stem cell field wakes up to hibernating cells
The definition of a true stem cell has long been thought to be that of a rare cell that preserves its long-term self-renewal potential and avoids compromising its genetic material by remaining inert. More recently, we have come to appreciate that different tissues harbor different stem cells that suit their requirements, including cycling and non-cycling stem cells (1). The existence of quiescent stem cells has been reported in many tissues, but until recently, such a stem cell has not been convincingly shown in the mammary gland. In a recent issue of Cell Stem Cell, Cai and colleagues [2017] reported the identification of a quiescent stem cell that expresses high levels of Bcl11b and is required for long-term maintenance of the mammary gland (2).
The mammary gland exists as a ductal tree composed of an inner luminal cell layer and outer layer of basal cells that contact the basement membrane and underlying stroma. The luminal compartment consists of luminal progenitors and more committed ductal luminal cells as well as alveolar cells that produce milk upon maturation during late pregnancy. The basal layer is composed of myoepithelial cells, whose contractile activity facilitates milk expulsion from the alveolar cells into the lumen and along mammary ducts. The basal layer is also thought to be where mammary stem cells reside in situ, although the niche remains evasive. Early work showed that any part of the mammary gland could engraft in recipient mice and reconstitute a new fully functional mammary gland, which could in turn be serially transplanted—suggesting the existence of mammary stem cells throughout the mammary epithelium (3-5). In comparison to other tissues, the best example of which is the haematopoietic system, the mammary gland epithelial hierarchy remains incompletely defined. However, much progress over the last decade using flow cytometry has led to the characterisation of the mammary epithelium into a hierarchy consisting of mature luminal or myoepithelial cells and the progenitors from which they arise, and a rare mammary stem cell at the apex (6-8). These stem cells are found in a population marked by the absence of lineage markers (Lin-) and the expression of CD24 and high levels of CD29 (6) or CD49f (9) cell surface markers. While this population (also called the basal cell subset) was shown to be enriched for mammary stem cell (MaSC) or mammary repopulating (MRU) activity, current sorting strategies define a highly heterogeneous population (10,11). Moreover, in recent years the use of reporter mice has led to significant debate in the field over the presence of unipotent or bipotent basal and luminal progenitors (7,8), however since these cells still show relatively high levels of proliferation as assessed by clonal analysis, they may represent a subset of cells downstream from a dormant stem cell.
Cai and colleagues now demonstrate that high levels of Bcl11b mark a quiescent stem cell population that has potent regenerative activity (2). In addition, the authors show that Bcl11b induces cells into G0 of the cell cycle and that loss of Bcl11b impairs normal mammary gland development. Furthermore, the authors suggest that regardless of whether bipotent or unipotent stem cells exist in the mammary hierarchy, Bcl11bhigh cells belong to a quiescent compartment upstream of more committed progenitors (Figure 1) and so are compatible with both models.
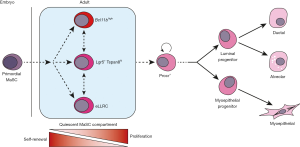
Cai and colleagues identified Bcl11b using single cell PCR on a small subset of CD49fhighCD24medLin- basal cells called Basal 1 cells, which were previously shown to be enriched for proliferating cells (10). Bcl11b expression was detected exclusively in basal cells that also express Krt17, although the significance of this at the protein level remains unclear. Using a reporter mouse, the authors find that Bcl11bhigh cells localise to the interface of basal and luminal cells, throughout the adult mammary gland, and are relatively rare, representing only 5% of the CD49fhighCD24medLin- subset.
What is the function of Bcl11b? Bcl11b is a zinc finger transcription factor shown to interact with several chromatin remodelling complexes and is required for the normal development of a number of tissues including T cells, skin and neurons (14). Upon deletion of Bcl11b using K14cre, Cai and colleagues observed a delay in mammary gland outgrowth suggesting that also in this tissue, Bcl11b is required for normal development. However, while many of the surviving cells in K14cre-Bcl11bfl/fl mammary epithelium had selected against Bcl11b deletion, some Bcl11b-deleted cells could still contribute to the developing mammary gland. This suggests that Bcl11b is not the only regulator of quiescent stem cells and/or that there is a degree of plasticity in the epithelial hierarchy so that other cells can be recruited to form the ductal epithelium in the absence of Bcl11b. Nevertheless, Bcl11b mutant cells had a lower frequency of regeneration capacity in recipient mice in primary transplantation assays, and failed to regenerate new mammary outgrowth upon secondary transplants. These findings suggest that deletion of Bcl11b from birth has long-lasting effects on the stem cell compartment. Furthermore, acute deletion of Bcl11b in adult mammary epithelial cells also reduced regeneration potential, highlighting the requirement for Bcl11b in maintaining adult mammary stem cells. It is worth noting however, that while transplantation assays have been extensively used to assess cell fate and stem cell activity, they may not accurately reflect the behaviour of unmanipulated cells [reviewed in (15)].
Since Bcl11bhigh cells gave rise to more colonies in in vitro assays of stem/progenitor cell activity, and had a higher engraftment in mice than Bcl11blow cells, the authors suggest that the effect of Bcl11b is cell intrinsic and conclude that these cells have a high regenerative activity. On the other hand, the authors observed that Bcl11bhigh cells rarely express Ki67 and instead found that Bcl11b was highly expressed in a Pyronin-low, Hoechst-low population, consistent with Bcl11b expression in label-retaining quiescent cells in G0. These observations are consistent with Bcl11b marking a quiescent cell with self-renewal properties.
To understand if and how Bcl11b may be suppressing mammary cell proliferation, the authors performed gain and loss of function assays. Microarray gene expression analysis revealed that Bcl11b-low cells were enriched for genes that regulate early G1 phase to prevent cell cycle progression, compared with cells overexpressing Bcl11b, but it is unclear if Bcl11b is directly or indirectly targeting these genes. Further, deletion of Bcl11b in organoid assays led to increased colony size, and a preliminary analysis of a R26creERT2 Bcl11bfl/fl mTmG mouse indicated the expansion of a Bcl11b-deleted basal population. While the authors interpret this as evidence that Bcl11b loss allows cells to leave a quiescent state, analysis of Pyronin and Hoechst expression, as well as serial passaging or transplantation assays would confirm if Bcl11b-deleted cells undergo earlier “exhaustion”. It is also of interest if an expanded basal population is observed in the mammary epithelium of Krt14cre-Bcl11bfl/fl mice.
In addition to the steady-state, the authors reported that Bcl11b expression decreased in response to pregnancy hormones to allow rapid proliferation. Bcl11b expression decreased in the CD49fhighCD24medLin- basal subset in pregnant mammary glands, however since Bcl11b-expressing cells represent only 5% of this population and the authors do not report if the size of this population changes during pregnancy, these findings are difficult to interpret.
Finally, the authors suggest that the regulation of cell cycle by Bcl11b may be partly mediated by p21, since p21-deficiency impaired the ability of Bcl11b overexpression to repress proliferation of CD49fhighCD24medLin- cells. Knockdown of Ink4A (Cdkn2a) also partially rescued the ability of Bcl11b-knockout cells to produce outgrowths in transplantation assays, suggesting that Bcl11b maintains quiescence partly through evasion of Cdkn2a-mediated senescence. Nevertheless, it would be important to see if Cdkn2a is also upregulated in Bcl11b-knockout cells from Krt14cre-Bcl11bfl/fl mice.
The findings of Cai et al., suggest the existence of quiescent stem cells in which Bcl11b-mediation suppression of senescence maintains their regenerative potential. How do the findings of Cai and colleagues compare with other work describing putative mammary stem cells? The authors showed that Bcl11b marks a cell distinct from the multipotent stem cells marked by protein C receptor (Procr) (11). Future work is required to determine how the Bcl11bhigh population is related to recently described quiescent stem cells that are marked by Lgr5 and Tspan8 expression and are spatially restricted to the proximal area of the mammary gland (12) and if it relates to spatially restricted stem cells that are laid down during embryogenesis (13). The existence of a quiescent mammary population is reminiscent of other tissues such as the haematopoietic system (16) and skin (17), where a quiescent compartment has evolved to ensure the integrity of the genetic code. However, their long life makes quiescent stem cells susceptible to genetic changes accumulated over time. It is therefore tantalizing to imagine what role Bcl11bhigh cells may have in breast cancer. Recently, high expression of the functionally related protein Bcl11a was reported in Triple-negative breast cancers (18). A similar analysis of Bcl11b expression in breast cancer subtypes and comparison with a Bcl11bhigh signature could provide some hints whether Bcl11b contributes to breast tumorigenesis.
The findings of Cai and colleagues raise a number of questions. The differences in the effect of Bcl11b deletion in vivo and in vitro warrants further investigation: acute Bcl11b deletion was reported to expand the basal cell compartment in vivo, but deletion from birth resulted in delayed mammary outgrowth – the composition of these glands was not reported, so a comparison is difficult to make here – it may be that cells that develop in the prolonged absence of Bcl11b have reduced fitness due to limiting niche/growth factors in vivo. Moreover, since acute Bcl11b deletion results in larger colonies in vitro, presumably due to absence of a quiescence signal, serial passaging could provide evidence of stem cell “exhaustion”. While the authors reported that pregnancy hormones downregulate Bcl11b, clonal analysis of reporter mice after several rounds of pregnancy and involution would confirm if Bcl11bhigh cells do not contribute to replenishing long-lived progenitors in post-involution mammary gland (8). Moreover, lineage tracing is critical to determine if Bcl11b marks a unipotent or bipotent progenitor.
At the molecular level, how is Bcl11b regulating target gene expression? Are genes such as p21 and cdkn2a direct targets of transcription? Bcl11b is reported to associate with a variety of cofactors, including the chromatin remodelling complexes NuRD (19) and SWI/SNF (20), as well as the histone acetyltransferase (HAT) p300 (21), functioning both as a transcriptional repressor and activator in a cell and context-dependent manner. It will be interesting to determine if like in T cell development, Bcl11b has cell and context-specific functions in the mammary epithelium.
Finally, it remains to be seen if Bcl11b mRNA expression translates to protein expression and whether like Tspan8 (12), Bcl11b or Krt17 expression could be useful as markers for the prospective isolation of mammary epithelial stem cells.
Acknowledgements
The breast cancer laboratory is supported by the Australian National Health and Medical Research Council (NHMRC) grants No. 1016701, No. 1024852, No. 1086727; NHMRC IRIISS; the Victorian State Government through VCA funding of the Victorian Breast Cancer Research Consortium and Operational Infrastructure Support; and the Australian Cancer Research Foundation. E.M.M. is supported by a National Breast Cancer Foundation Career Development Fellowship. Thanks to Jane Visvader for critical reading of the manuscript and Peter Maltezos for assistance with preparation of figures.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Visvader JE, Clevers H. Tissue-specific designs of stem cell hierarchies. Nat Cell Biol 2016;18:349-55. [Crossref] [PubMed]
- Cai S, Kalisky T, Sahoo D, et al. A quiescent Bcl11b high stem cell population is required for maintenance of the mammary gland. Cell Stem Cell 2017;20:247-260.e5. [Crossref] [PubMed]
- Daniel CW, De Ome KB, Young JT, et al. The in vivo Life span of normal and preneoplastic mouse mammary glands: a serial transplantation study. Proc Natl Acad Sci U S A 1968;61:53-60. [Crossref] [PubMed]
- Hoshino K. Morphogenesis and growth potentiality of mammary glands in mice. I. Transplantability and growth potentiality of mammary tissue of virgin mice. J Natl Cancer Inst 1962;29:835-51. [PubMed]
- Smith GH, Medina D. A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J Cell Sci 1988;90:173-83. [PubMed]
- Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature 2006;439:84-8. [Crossref] [PubMed]
- Van Keymeulen A, Rocha AS, Ousset M, et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature 2011;479:189-93. [Crossref] [PubMed]
- Rios AC, Fu NY, Lindeman GJ, et al. In situ identification of bipotent stem cells in the mammary gland. Nature 2014;506:322-7. [Crossref] [PubMed]
- Stingl J, Eirew P, Ricketson I, et al. Purification and unique properties of mammary epithelial stem cells. Nature 2006;439:993-7. [PubMed]
- Prater MD, Petit V, Alasdair Russell I, et al. Mammary stem cells have myoepithelial cell properties. Nat Cell Biol 2014;16:942-50, 1-7.
- Wang D, Cai C, Dong X, et al. Identification of multipotent mammary stem cells by protein C receptor expression. Nature 2015;517:81-4. [Crossref] [PubMed]
- Fu NY, Rios AC, Pal B, et al. Identification of quiescent and spatially restricted mammary stem cells that are hormone responsive. Nat Cell Biol 2017;19:164-76. [Crossref] [PubMed]
- Boras-Granic K, Dann P, Wysolmerski JJ. Embryonic cells contribute directly to the quiescent stem cell population in the adult mouse mammary gland. Breast Cancer Res 2014;16:487. [Crossref] [PubMed]
- Kominami R. Role of the transcription factor Bcl11b in development and lymphomagenesis. Proc Jpn Acad Ser B Phys Biol Sci 2012;88:72-87. [Crossref] [PubMed]
- Visvader JE, Stingl J. Mammary stem cells and the differentiation hierarchy: current status and perspectives. Genes Dev 2014;28:1143-58. [Crossref] [PubMed]
- Eaves CJ. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood 2015;125:2605-13. [Crossref] [PubMed]
- Alcolea MP, Jones PH. Lineage analysis of epidermal stem cells. Cold Spring Harb Perspect Med 2014;4:a015206. [Crossref] [PubMed]
- Khaled WT, Choon Lee S, Stingl J, et al. BCL11A is a triple-negative breast cancer gene with critical functions in stem and progenitor cells. Nat Commun 2015;6:5987. [Crossref] [PubMed]
- Cismasiu VB, Adamo K, Gecewicz J, et al. BCL11B functionally associates with the NuRD complex in T lymphocytes to repress targeted promoter. Oncogene 2005;24:6753-64. [Crossref] [PubMed]
- Kadoch C, Hargreaves DC, Hodges C, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet 2013;45:592-601. [Crossref] [PubMed]
- Cismasiu VB, Ghanta S, Duque J, et al. BCL11B participates in the activation of IL2 gene expression in CD4+ T lymphocytes. Blood 2006;108:2695-702. [Crossref] [PubMed]
Cite this article as: Michalak EM. The mammary stem cell field wakes up to hibernating cells. Stem Cell Investig 2017;4:45.


