Fetal hematopoietic stem cells are making waves
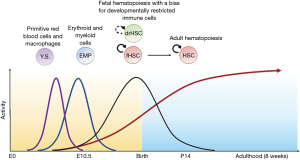
Hematopoiesis first arises during early embryogenesis when passive diffusion of oxygen and nutrients becomes insufficient to support the developing organism. Early hematopoietic development is largely focused on the production of red blood cells and tightly linked to concomitant vascular development. As a result of their coordinated functions, both the hematopoietic and cardiovascular systems are essential for fetal survival, unlike other organ systems. Beyond these initial functions, the fetal hematopoietic system evolves to generate a diverse output of mature elements, including erythrocytes, platelets, macrophages, other myeloid cells, innate lymphocytes, as well as T and B lymphocytes arising from progenitors that have gained the capacity to rearrange antigen receptor genes. During fetal life, subsets of hematopoietic progenitors acquire long-term self-renewal potential as assessed after transplantation into lethally irradiated hosts, a functional property that has been used historically to define hematopoietic stem cells (HSCs) (1-4). Fetal HSCs are thought to seed the more quiescent adult HSC compartment that will sustain the adult hematopoietic system, a process taking place in the bone marrow after it becomes available to support hematopoiesis (5-7). In parallel, fetal hematopoiesis generates subsets of macrophages and lymphocytes that are uniquely produced during fetal life, suggesting that they play a role in shaping the developing immune system’s reactivity. How these complex processes and developmental transitions are regulated has been the subject of intense scrutiny. In the prevailing model, fetal hematopoiesis occurs in distinct “waves” that take place during a defined developmental span and perform a restricted set of hematopoietic functions (8,9) (Figure 1). However, it remains unclear how these physiological transitions are coupled to the emergence of HSCs with transplantation potential, and with the transition from the fetal to the adult HSC compartment.
In a recent issue of Cell Stem Cell, Beaudin and coworkers add a new twist to our understanding of fetal hematopoiesis by identifying and characterizing a subset of fetal HSCs that are endowed with long-term self-renewal potential in transplantation assays (thus qualifying as bona fide HSCs), but that fail to persist into adulthood in physiological conditions (10). During their natural life, these developmentally restricted HSCs (drHSCs) appear biased to produce B and T lymphocytes with innate-like characteristics, providing a first line of defense to the developing organism. Paradoxically, drHSCs sustain long-term lymphoid-biased multilineage reconstitution after transplantation, but not in physiological conditions, suggesting that they have the plasticity to activate a latent self-renewal potential only when exposed to stress conditions. These observations suggest that HSC potential as defined in transplantation assays does not necessarily correlate with long-term persistence of the cells in their native environment.
What enabled Beaudin and coworkers to make these new observations? Their work is rooted in longstanding efforts by multiple laboratories to characterize cell surface markers that distinguish true long-term HSCs from downstream progenitors, and in work from the Forsberg laboratory to capitalize on this information with genetic lineage tracing systems. In adult hematopoietic stem and progenitor cells, expression of the tyrosine kinase receptor Flk2 (also known as Flt3, CD135) is tightly associated with loss of self-renewal potential as progenitors exit the HSC compartment (10-12). In addition, high levels of Flk2 expression correlate with the activation of a genetic program that supports early stages of lymphoid development, a process also known as lymphoid priming (13-15). To build on these findings, Forsberg and collaborators developed a Flk2-Cre allele to lineage trace cells that have a history of Flk2 expression. Combining this allele with a ROSA26-driven mT/mG reporter system led to the generation of a “FlkSwitch” mouse model in which Flk2-Cre-expressing cells irreversibly excise the Tomato gene, while initiating GFP expression in these cells and all their progeny11. In FlkSwitch mice, adult HSCs were always Tomato positive (Tom+), consistent with the fact that developmental precursors of HSCs and HSCs themselves are Flk2 negative (11). In contrast, most mature hematopoietic cells were GFP+, suggesting that all blood lineages proceed through a state that involves Flk2 expression (11). In transplantation assays, Tom+ cells (presumably long-term and short-term HSCs) sustained durable multilineage reconstitution, while GFP+ progenitors only supported limited, short-term reconstitution. Altogether, this work suggested that Flk2 expression was associated with loss of self-renewal potential and initiation of robust proliferation in adult hematopoietic progenitors (10-12,14).
During fetal hematopoiesis, however, activity of the FlkSwitch system correlated very differently with HSC phenotype and transplantability as compared to adult hematopoiesis, giving rise to the identification of the fetal drHSCs recently reported by Beaudin and coworkers (10). When assessed during mid-gestation in FlkSwitch mice, phenotypically defined HSCs contained both Tom+ and GFP+ cells, with many cells expressing Tomato and GFP simultaneously, suggesting that they had only recently undergone Cre-mediated switch to GFP expression. This was a surprising result, as previous work showed that adult HSCs were always Tom+, while GFP+ cells were presumed to be multipotent progenitors with limited self-renewal potential. GFP+ cells with HSC phenotypic characteristics were detected up to postnatal day 14 in the bone marrow, around the time at which functional transition from rapidly cycling fetal-like to quiescent adult HSCs was previously described (5,6). Importantly, Beaudin et al. did not limit their investigation to phenotypic markers, but studied in detail the functional potential of Tom+ and GFP+ fetal hematopoietic progenitors when transplanted into irradiated adult recipients. While Tom+ hematopoietic progenitors were the only adult cells capable of long-term reconstitution, both fetal Tom+ and GFP+ progenitors were capable of long-term reconstitution across multiple lineages in primary and even in secondary transplantation recipients, consistent with their long-term self-renewal potential and with their classification as HSCs. Fetal GFP+ HSCs were equally potent as Tom+ HSCs to reconstitute lymphoid lineages, although their contribution to myeloid and erythroid/megakaryocytic lineages was lower. Altogether, fetal GFP+ HSCs had a lower progenitor frequency when using trilineage reconstitution as a readout in limiting dilution transplantation assays. These findings suggested a lymphoid bias of the fetal GFP+ HSCs and correlated with upregulated expression of multiple lymphoid priming genes in these cells as compared to Tom+ HSCs (e.g., Rag1, Rag2, Il7ra, Ccr9). Moreover, fetal GFP+ HSCs demonstrated not only an overall lymphoid bias in their lineage output, but also a specific bias towards defined subsets of lymphocytes with innate-like characteristics that are produced predominantly during fetal life (e.g., B1a B cells and Vγ3+ TCR-γδ T cells, at least as assessed experimentally in a fetal thymic microenvironment). Although many other cellular sources could also be involved, these findings suggest that the newly identified GFP+ drHSCs contribute to the potent and unique lymphoid output that characterizes the fetal hematopoietic system.
When tracking GFP+ HSCs during ontogeny in FlkSwitch mice, Beaudin et al. uncovered what can be considered the central paradox of their discovery: despite their potent latent self-renewal potential as revealed in transplantation assays, GFP+ HSCs naturally persisted only shortly after birth, and they were completely absent from the adult bone marrow (Figure 1). These findings were further validated experimentally by in utero transplantation of Tom+vs. GFP+ fetal HSCs, demonstrating that in these conditions and in the absence of host irradiation, only Tom+ but not GFP+ HSCs gave rise to potent and persistent multilineage chimerism once the transplanted embryos reached adulthood. These findings are consistent with the coexistence of at least two populations of fetal HSCs: a population of “conventional” fetal HSCs that eventually transitions into adult HSCs and seeds the adult hematopoietic system; and a population of drHSCs that is programmed for disappearance, unless introduced experimentally into irradiated adult recipients. These observations suggest the existence of plasticity in drHSCs to activate a latent self-renewal program in the post-transplantation setting, when these cells are exposed to environmental cues that are absent in their natural environment. Of note, functional, phenotypic and gene expression analysis revealed that conventional Tom+ fetal HSCs were closer to adult HSCs than GFP+ drHSCs, for example in terms of their relative quiescence (a characteristic feature of adult HSCs in steady-state conditions).
What allows fetal but not adult HSCs to maintain self-renewal potential after they turn on Flk2 expression? One interesting observation in FlkSwitch mice is that fetal hematopoietic progenitors activating the Flk2-Cre-driven switch to GFP expression nearly always coexpressed Tomato, suggesting that the switch had happened only very recently and that preformed Tomato protein had not been degraded yet. In contrast, most adult GFP+ progenitors had already lost Tomato expression, suggesting that they were captured after a much longer exposure to functional changes that are induced upon Flk2 transcriptional activation. Interestingly, hematopoietic progenitors found in early post-natal bone marrow (P14) displayed both Tom+/GFP+ and GFP+ subsets, which could be an opportunity to test in the future if the HSC self-renewal potential clusters exclusively with the “younger” Tom+/GFP+ cells. A second consideration is that drHSCs appeared to generate a population of Flk2– HSCs after transplantation into irradiated adult recipients. Although not tested formally, it is tempting to speculate that this capacity to turn off Flk2 expression was linked to the acquisition of long-term and serial repopulation potential in adult recipients. Thus, it is possible that epigenetic remodeling at the Flk2 locus differs in fetal and adult HSCs after they first activate Flk2 expression, with more plasticity in fetal HSCs. It is also possible that Flk2-mediated signals are different in fetal and adult hematopoietic progenitors in terms of their intensity or in terms of their association with other gene expression programs. Finally, fetal and adult HSCs may simply be wired differently in terms of their tolerance to Flk2-mediated and other trophic signals. For example, Pten loss (which activates signals downstream of PI3K/Akt) causes proliferation followed by profound depletion of adult HSCs, while fetal HSCs can tolerate Pten inactivation, and fetal/neonatal but not adult HSCs are resistant to the effects of Flk2/Flt3 activation by the leukemia-associated protein Flt3-ITD (Flt3 with an internal tandem duplication) (16,17).
Although transplantation assays have been instrumental to rigorously and prospectively define HSCs, findings by Beaudin and coworkers highlight the fact that transplantation into irradiated recipients remains an experimental assay, and that behavior of a given population of progenitors after transplantation (e.g., long-term reconstitution from drHSCs) does not necessarily match their in vivo activity (e.g., spontaneous disappearance of these cells in physiological conditions). Conversely, recent work questioned the extent to which adult HSCs contribute to steady-state hematopoietic output as compared to downstream multipotent or oligopotent progenitors that have only limited reconstitution potential in transplantation assays (18,19). Although these questions remain intensely debated, and adult HSCs may still be essential to sustain much of life-long hematopoiesis (20,21), they bring a healthy perspective into the field by reminding us that HSC transplantability and long-term reconstitution potential, although useful and trackable, remain very artificial measurements that do not represent the natural conditions to which hematopoietic progenitors are being exposed.
The identification of drHSCs should also be put into perspective in terms of how the developing hematopoietic system is organized. In principle, nature could have opted for a linear system in which each stage of fetal hematopoiesis transitions into the next one, with changing functional characteristics at different time points. For many years, investigators have debated whether such a transition happens between the “primitive” hematopoiesis that first emerges in the extraembryonic yolk sac and subsequent “definitive” hematopoiesis arising at intraembryonic sites (e.g., in the floor of the abdominal aorta and in association with other arterial vessels). However, classical experiments performed in avian embryo chimeras provided conclusive evidence that the primitive wave of extraembryonic hematopoiesis is distinct from subsequent fetal and adult hematopoiesis: quail embryos implanted into a chick yolk sac exhibited persistent quail-derived hematopoiesis, despite functional connections between the embryonic and the yolk sac vasculature (22). Today, most investigators agree that primitive and definitive waves of hematopoiesis are similarly distinct in mammalian organisms (8,9). Moreover, additional transient waves of hematopoietic progenitors have been reported, including the Runx1-dependent erythro-myeloid progenitors that function in mid-gestation in mice (8). The newly discovered drHSCs fit within this organizational principle by sustaining hematopoiesis only transiently and with biased functions to meet unique temporal needs of the developing organism. Thus, the hematopoietic system that prevails at different stages of ontogeny results from the successive output of multiple distinct developmental waves—very much like a painting drawn on a canvas that contained earlier iterations of the artist’s work.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lorenz E, Uphoff D, Reid TR, et al. Modification of irradiation injury in mice and guinea pigs by bone marrow injections. J Natl Cancer Inst 1951;12:197-201. [PubMed]
- Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science 1988;241:58-62. [Crossref] [PubMed]
- Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 1963;197:452-4. [Crossref] [PubMed]
- Eaves CJ. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood 2015;125:2605-13. [Crossref] [PubMed]
- Bowie MB, Kent DG, Dykstra B, et al. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci U S A 2007;104:5878-82. [Crossref] [PubMed]
- Bowie MB, McKnight KD, Kent DG, et al. Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. J Clin Invest 2006;116:2808-16. [Crossref] [PubMed]
- Jones M, Chase J, Brinkmeier M, et al. Ash1l controls quiescence and self-renewal potential in hematopoietic stem cells. J Clin Invest 2015;125:2007-20. [Crossref] [PubMed]
- Chen MJ, Li Y, De Obaldia ME, et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 2011;9:541-52. [Crossref] [PubMed]
- Lacaud G, Kouskoff V. Hemangioblast, hemogenic endothelium, and primitive versus definitive hematopoiesis. Exp Hematol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Beaudin AE, Boyer SW, Perez-Cunningham J, et al. A Transient Developmental Hematopoietic Stem Cell Gives Rise to Innate-like B and T Cells. Cell Stem Cell 2016;19:768-83. [Crossref] [PubMed]
- Boyer SW, Schroeder AV, Smith-Berdan S, et al. All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell Stem Cell 2011;9:64-73. [Crossref] [PubMed]
- Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A 2001;98:14541-6. [Crossref] [PubMed]
- Adolfsson J, Månsson R, Buza-Vidas N, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 2005;121:295-306. [Crossref] [PubMed]
- Sitnicka E, Buza-Vidas N, Ahlenius H, et al. Critical role of FLT3 ligand in IL-7 receptor independent T lymphopoiesis and regulation of lymphoid-primed multipotent progenitors. Blood 2007;110:2955-64. [Crossref] [PubMed]
- Ng SY, Yoshida T, Zhang J, et al. Genome-wide lineage-specific transcriptional networks underscore Ikaros-dependent lymphoid priming in hematopoietic stem cells. Immunity 2009;30:493-507. [Crossref] [PubMed]
- Magee JA, Ikenoue T, Nakada D, et al. Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell 2012;11:415-28. [Crossref] [PubMed]
- Porter SN, Cluster AS, Yang W, et al. Fetal and neonatal hematopoietic progenitors are functionally and transcriptionally resistant to Flt3-ITD mutations. Elife 2016;5:e18882. [Crossref] [PubMed]
- Busch K, Klapproth K, Barile M, et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 2015;518:542-6. [Crossref] [PubMed]
- Sun J, Ramos A, Chapman B, et al. Clonal dynamics of native haematopoiesis. Nature 2014;514:322-7. [Crossref] [PubMed]
- Sawai CM, Babovic S, Upadhaya S, et al. Hematopoietic Stem Cells Are the Major Source of Multilineage Hematopoiesis in Adult Animals. Immunity 2016;45:597-609. [Crossref] [PubMed]
- Schoedel KB, Morcos MN, Zerjatke T, et al. The bulk of the hematopoietic stem cell population is dispensable for murine steady-state and stress hematopoiesis. Blood 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Dieterlen-Lievre F. On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J Embryol Exp Morphol 1975;33:607-19. [PubMed]
Cite this article as: Waas B, Maillard I. Fetal hematopoietic stem cells are making waves. Stem Cell Investig 2017;4:25.


