Peptide vaccination against multiple myeloma using peptides derived from anti-apoptotic proteins: a phase I trial
One hallmark of multiple myeloma (MM) is lacking apoptosis. Notably the B-cell lymphoma-2 (Bcl-2) family of proteins play central roles in the regulation of apoptosis and are frequently overexpressed in cancer, including MM (1). The Bcl-2 family comprises anti-apoptotic as well as pro-apoptotic proteins, with the anti-apoptotic proteins including both Bcl-2 and the Bcl-2-like proteins Bcl-XL, Bcl-w, Mcl-1 and A1 (2). Bcl-2 and Bcl-2-like anti-apoptotic proteins are promising targets for immunotherapy: if clones escape the effects of therapy by downregulating Bcl-2, it will hinder the sustained neoplastic growth. Our group reported spontaneous T-cell reactivity against Bcl-2, Bcl-XL and Mcl-1 in peripheral blood from patients with different unrelated cancers (3-5). We have furthermore shown that T cells specific to peptide epitopes from these three proteins can kill HLA-matched tumor cells from a number of cancer types (3,6,7). The Mcl-1 protein plays a vital role in MM-cell survival (8). However, gene expression of the Bcl-2 family members differs among MM molecular subgroups (9). Proteasome inhibitors have been found to improve the presentation of exogenous peptides on dendritic cells. This suggests that concomitant proteasome inhibition would lead to an improved immunogenicity of synthetic peptide vaccination (10). Here we report a phase I trial of vaccination using HLA-restricted nonamer to decamer peptides from the Bcl-2, Bcl-XL and Mcl-1 proteins concomitant with bortezomib in patients with relapsed MM. The primary endpoint was assessment of toxicity and safety.
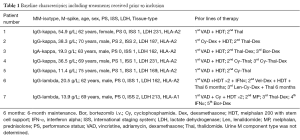
Patients were eligible for inclusion if they had relapsed from verified MM and, at the time of inclusion they were not candidates for high dose melphalan with autologous stem cell transplantation (HDT). The trial was set up to be a phase I/II trial, including initially 6 patients, with a possibility to continue to a phase II with initially 14 patients, with a possibility to include a total of 40 patients if at least one of the initial 14 patients responded. The vaccine dose was fixed with no dose escalation. Patients were enrolled in the years 2010 and 2011. After enrollment of 7 patients, bortezomib with dexamethasone became a standard therapy for the treatment population, and no further patients could be recruited. Due to peptide HLA-restriction, patients received peptides according to their HLA-A-positivity, i.e., HLA-A1, HLA-A2 and/or HLA-A3. Patients could be included if their performance status (PS) was 2 or better and an expected survival of more than 3 months. Sufficient bone-marrow function was required (leucocytes >2.5×109/L, granulocytes >1.5×109/L and thrombocytes >50×109/L), as did renal function (p-creatinine <2.5 times the upper normal limit) and liver function (ASAT <100 IU, Bilirubin <30 IU). Patients with other active malignancies, other significant comorbidities, acute or chronic infection with HIV, hepatitis or tuberculosis, serious allergies, active autoimmune diseases or uncontrolled hypercalcemia were excluded. Treatment with immunosuppressive drugs, including steroids, and systemic antineoplastic treatment was not permitted. Patients were allowed to receive bisphosphonates. The Ethical Committee of the Region of Southern Denmark, the Danish data Protection Agency, and the Danish Medical Agencies (EudraCT 2006-003619-29) approved the study and all patients signed a written consent form. The trial was conducted in accordance with the Helsinki declaration and Good Clinical Practice (GCP) and monitored by the GCP-unit, Region of Southern Denmark, Denmark. Patients were enrolled and treated at the Departments of Haematology at Herlev Hospital (Herlev, Denmark) and Odense University Hospital (Odense, Denmark).
Peptides were derived from unmodified wildtype Bcl-2 family proteins and Montanide ISA-51 was used as adjuvant. The peptides were as follows: HLA-A1 restricted: Mcl-1 [166–175] PAEEEEDDLY, Mcl-1 [177–185] QSLEIISRY; HLA-A2 restricted: Bcl-2 [208–217] PLFDSWLSL, Bcl-2 [214–223] WLSLKTLLSL, Bcl-XL [173–182] YLNDHLEPWI, Bcl-XL [165–174] RIAAWMATYL; HLA-A3 restricted: Bcl-XL [165–173] RIAAWMATY, Mcl-1 [95–103] RLLFFAPTR, Mcl-1 [300–308] RTKRDWLVK. The peptides were synthesized at >95% purity by an external manufacturer (PolyPeptide, France). Before use, the peptides were suspended in PBS/DMSO, mixed and sterile-filtered through a 0.22-micron filter. Endotoxin- and culture tests were then performed. Brief description of the procedure: just prior to injection, 300 µL of the peptide mixture corresponding to the HLA type was mixed with 700 µL Montanide ISA-51. The peptide mixture contained 333 µg/mL of each peptide in the HLA-restricted group suspended in PBS/30% DMSO. This was mixed using a whirl mixer for 5 min and then administered as a deep subcutaneous injection. Patients were monitored for acute toxicity for 2 h after the injection. Up to 1,000 mg paracetamol could be offered as a mild painkiller as needed. The patients were vaccinated a total of 8 times during 4 series of bortezomib treatment. Bortezomib was given in 21-day series, with bortezomib intravenous injections on days 1, 4, 8 and 11 followed by a 10-day treatment break. Vaccinations were given on days 2 and 9. One patient (patient 3) achieved a partial remission (PR) and continued with monthly maintenance vaccinations without bortezomib.
Blood samples were drawn before start of treatment and at the time of every vaccination. Bone marrow biopsies were performed after 4 and 8 vaccinations and then 3 months after the last vaccination. Thereafter, bone marrow biopsies were performed at the treating physician’s discretion.
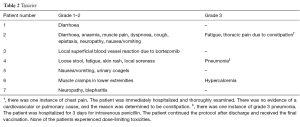
To assess whether vaccination resulted in a measurable T-cell response, we performed indirect IFN-γ-ELISPOT as described by McCutcheon et al. (11). In brief, nitrocellulose-bottomed 96-well plates (Multiscreen MAIP N45; Millipore) were coated overnight with the relevant antibody, washed, blocked with X-vivo and incubated for 2 h. The blocking agent was removed and 200 µL of cells ± peptide was added and incubated in the plate overnight. The next day, the plates were washed, and the relevant secondary biotinylated antibody (Mabtech) was added. After 2 h, the plates were washed and streptavidin (AP-avidin; Calbiochem/Invitrogen Life Technologies) was added and incubated 1 hour. After washing, the assay was developed by adding the enzyme substrate NBT/BCIP (Invitrogen Life Technologies) for 1–2 min. The spots were counted using the ImmunoSpot Series 2.0 Analyzer (CTL Analyzer). The response defined was based on the guidelines and on the recommendations provided by the CIP working group as well as Moodie and colleagues (12). Baseline characteristics of patients included are summarized in Table 1. Toxicity is summarized in Table 2.
Full table
Full table
The ELISPOT data for the peripheral blood (PB) immune reactivity to the peptides were available for 6 of the 7 patients. One patient (number 6) dropped out after 2 vaccinations; we do not have data from that patient. The quality of the bone marrow samples was not good enough for ELISPOT assessment. All patients showed immune responses in PB to the peptides. Out of the 6 evaluable patients, 3 showed signs of increased immune reactivity after vaccination (patients 3, 4 and 5). Specifically, these patients showed increased levels of INF-γ release in the post-vaccination ELISPOT assay compared to baseline. Unfortunately, blood samples of sufficient quality were limited and analyses could therefore only be carried out in singlets. Figure 1 shows representative ELISPOT data from patient 4.
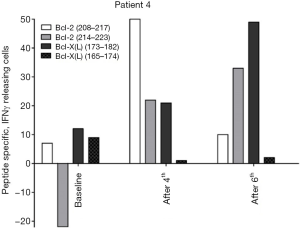
Patient 2 showed signs of a decreased immune response in the peripheral blood. This patient received the full 8 vaccinations, but three months after the last vaccination the patient experienced progressive disease and died from that relapse.
This study showed that it was feasible and safe to vaccinate MM patients with peptides from the Bcl-2 family in Montanide during treatment with bortezomib in a relapsed and refractory setting. Out of an intention-to-treat population of 7 patients, 4 patients completed the 8 vaccinations that were given along with 4 series of bortezomib. One of these patients continued to receive monthly maintenance vaccinations for 9 months before progressing and switching to another therapy.
Toxicity was mild and not different from toxicity expected with the use of intravenous bortezomib. The three patients not completing 8 vaccinations went off-study due to objective progression, clinical decision of lacking effect and development of hypercalcemia, respectively. None of the patients showed signs of auto-reactivity.
Immuno-monitoring demonstrated vaccination-induced peptide antigen-specific T-cell responses in 3 patients, but the sample quality was not sufficient to verify these responses further. The data are too limited to assess clinical efficacy of the vaccinations, but out of 3 patients with immune response, 2 completed the vaccination protocol, 1 of whom had a partial response and went on to have 9 monthly maintenance vaccinations before developing progressive disease.
Immune reactivity can be assessed using several different methods. Tetramer assays can be used to identify peptide specific T cells (13). This method handles the low affinity of monomeric TCR-MCH binding by coupling 4 biotinylated peptide-MHC-molecules to one streptavidin molecule, which is labelled with a fluorochrome. Immune reactivity can also be documented with a proliferation assay. These assays can be used to assess proliferation of T cells stimulated with an antigen, but they cannot contribute with functional assessment of the proliferating cells. A functional assessment can be achieved by several means, the simplest of which is the chromium release assay. In this cytotoxic assay, target cells are labelled by being treated with radioactive sodium chromate. Cells release sodium chromate when they are killed. Thereby the level of killing performed by, e.g., T-cells against labelled malignant target cells can be assessed by measuring the radioactivity of the supernatant. In the present study, we unfortunately only had material to perform the ELISPOT assays as described.
Looking ahead, additional trials of peptide vaccination against anti-apoptotic targets would still be very interesting. Due to its immunosuppressive effects dexamethasone is generally thought to be difficult to combine with therapeutic vaccination; however, interestingly, dexamethasone seems to increase Bcl-2 dependence in MM resulting in increased sensitivity to the Bcl-2-inhibitor venetoclax in vitro (14). Furthermore, a recent paper reported the efficacy of a peptide vaccine combined with low-dose dexamethasone in prostate cancer (15). This is interesting when considering future trials of peptide vaccinations in MM, since most MM treatments contain dexamethasone.
In the present trial, the peptides were nona- and decamers peptides. Longer peptides from the Bcl-2 family would be interesting alternative vaccination candidates. Earlier we observed spontaneous immune responses against long peptides derived from Bcl-XL in cancer patients (16). Long peptides have higher chances of containing several MHC-class I epitopes, which eliminates the HLA-restriction on patient enrolment. Furthermore, individual long peptides can contain both MHC-class I and MHC-class II epitopes, allowing T-helper stimulation to contribute to the effect. To this end, we are currently preparing to conduct a phase I trial that will use a 42-amino acid peptide derived from Bcl-XL with an adjuvant containing poly(I:C) for patients with prostate cancer.
Acknowledgements
We would like to thank Merete Jonassen for outstanding technical assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Punnoose EA, Leverson JD, Peale F, et al. Expression Profile of BCL-2, BCL-XL, and MCL-1 Predicts Pharmacological Response to the BCL-2 Selective Antagonist Venetoclax in Multiple Myeloma Models. Mol Cancer Ther 2016;15:1132-44. [Crossref] [PubMed]
- Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007;26:1324-37. [Crossref] [PubMed]
- Andersen MH, Svane IM, Kvistborg P, et al. Immunogenicity of Bcl-2 in patients with cancer. Blood 2005;105:728-34. [Crossref] [PubMed]
- Andersen MH, Becker JC, Thor Straten P. The antiapoptotic member of the Bcl-2 family Mcl-1 is a CTL target in cancer patients. Leukemia 2005;19:484-5. [Crossref] [PubMed]
- Andersen MH, Reker S, Kvistborg P, et al. Spontaneous immunity against Bcl-xL in cancer patients. J Immunol 2005;175:2709-14. [Crossref] [PubMed]
- Sørensen RB, Hadrup SR, Køllgaard T, et al. Efficient tumor cell lysis mediated by a Bcl-X(L) specific T cell clone isolated from a breast cancer patient. Cancer Immunol Immunother 2007;56:527-33. [Crossref] [PubMed]
- Sørensen RB, Nielsen OJ, Thor Straten P, et al. Functional capacity of Mcl-1-specific cytotoxic T-cells. Leukemia 2006;20:1457-8. [Crossref] [PubMed]
- Zhang B, Gojo I, Fenton RG. Myeloid cell factor-1 is a critical survival factor for multiple myeloma. Blood 2002;99:1885-93. [Crossref] [PubMed]
- Gomez-Bougie P, Amiot M. Apoptotic machinery diversity in multiple myeloma molecular subtypes. Front Immunol 2013;4:467. [Crossref] [PubMed]
- Chromik J, Schnürer E, Georg Meyer R, et al. Proteasome-inhibited dendritic cells demonstrate improved presentation of exogenous synthetic and natural HLA-class I peptide epitopes. J Immunol Methods 2006;308:77-89. [Crossref] [PubMed]
- McCutcheon M, Wehner N, Wensky A, et al. A sensitive ELISPOT assay to detect low-frequency human T lymphocytes. J Immunol Methods 1997;210:149-66. [Crossref] [PubMed]
- Moodie Z, Price L, Janetzki S, et al. Response determination criteria for ELISPOT: toward a standard that can be applied across laboratories. Methods Mol Biol 2012;792:185-96. [Crossref] [PubMed]
- Altman JD, Moss PA, Goulder PJ, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 1996;274:94-6. [Crossref] [PubMed]
- Matulis SM, Gupta VA, Nooka AK, et al. Dexamethasone treatment promotes Bcl-2 dependence in multiple myeloma resulting in sensitivity to venetoclax. Leukemia 2016;30:1086-93. [Crossref] [PubMed]
- Yoshimura K, Minami T, Nozawa M, et al. A Phase 2 Randomized Controlled Trial of Personalized Peptide Vaccine Immunotherapy with Low-dose Dexamethasone Versus Dexamethasone Alone in Chemotherapy-naive Castration-resistant Prostate Cancer. Eur Urol 2016;70:35-41. [Crossref] [PubMed]
- Larsen SK, Hansen M, Svane IM, et al. Characterization of Spontaneous Immune Responses against Long Peptides Derived from Bcl-X(L) in Cancer Patients Using Elispot. Cells 2012;1:51-60. [Crossref] [PubMed]
Cite this article as: Jørgensen NG, Ahmad SM, Abildgaard N, Straten PT, Svane IM, Andersen MH, Knudsen LM. Peptide vaccination against multiple myeloma using peptides derived from anti-apoptotic proteins: a phase I trial. Stem Cell Investig 2016;3:95.


