Epigenetic regulation of human DCLK-1 gene during colon-carcinogenesis: clinical and mechanistic implications
DCLK1 was first described in the late 1990s as a putative kinase, with homology to double-cortin (DCX), and the gene was mapped to chromosome 13q13 (1). Long (L) and short (S) variants of DCLK1 have been described, where in the L variants contain DCX domains, and the (S) variants lack the DCX domains (1). At the C-terminal end, DCLK1 has homology with Ca2+/calmodulin-dependent kinase (CAMK), but lacks calmodulin binding motifs, and is calmodulin-independent (2). The exon/intron borders of hDCLK1 gene were characterized (3), followed by characterization of mouse (m) Dclk1 variants (4). Significant differences in the mouse and human DCLK1 were identified (5), but in both human and mice, DCLK1 was reported to regulate neuronal migration (4). The DCX domains were required for association with microtubules, and the full-length (L) DCLK1 was required for polymerization and formation of microtubule structures (6,7). Till recently, it was widely believed that the S-isoforms of DCLK1 arose due to calpain-mediated cleavage (8). Possible use of an alternate (β) promoter for expressing shorter variants, however, had been speculated [as discussed in (9)]. While crystal structures of specific domains within DCLK1 (including DCX) have been resolved (10,11), substrates and regulators of DCLK1 have remained elusive, earning the molecule the term ‘orphan kinase’. Key role of DCLK1-L in neurogenesis, neuronal migration, cortical development and dendrite growth are by now well established (12,13). DCLK1 also influences cognitive traits such as memory and IQ scores (14). Loss of DCX domains in mice was reported to result in a more anxious behavioral phenotype (15).
The first evidence that DCLK1 is also expressed by epithelial cells came in 2006, when it was described as a stem cell marker in the stomach (16). Dclk1+ cells were located below the transit amplifying cells in the stomach mucosa, did not express differentiation markers, did not stain for BrdU, and were proposed as a marker of adult gut stem cells in mice (16). Studies by the groups of Anant and Houchen demonstrated that Dclk1 was expressed at the +4 position in intestinal crypts of mice and co-localized with another stem cell marker (MSI-1) (17). The Dclk1+ cells were negative for PCNA staining and termed quiescent stem cells (17,18). Importantly the authors demonstrated that Lgr5+ cells were PCNA positive while Dclk1+ cells were PCNA negative, suggesting Lgr5 represented actively proliferating stem cells (progenitors?), while Dclk1 represented +4 quiescent stem cells in the mouse intestinal crypts (18). The Dclk1+ cells grew as organoids in nude mice, suggesting Dclk1+ cells were pluripotent, a hallmark of stem cells (18). We and others reported a significant increase in the number and intensity of Dclk1+cells in hyper proliferating mouse colonic crypts, in response to potent growth factors (19) or colon carcinogenic agents, AOM ± DSS (20), suggesting a role of Dclk1+ cells, at position 4, in hyper proliferation and carcinogenesis of colonic crypt cells. However, it soon became clear that Dclk1 is also expressed in specialized cells, called tuft cells, in the mouse colonic crypts which are enriched in Cox1/Cox2, Villin and α-Tubulin, and believed to be derived from Lgr5+ actively cycling stem/progenitor cells (21). Dclk1+ cells in mouse intestinal crypts, were recently reported to represent both quiescent/cycling stem cells, including tuft cells, and reported to express pluripotency factors (Oct4, Sox2, Nanog and Klf4), and give rise to intestinal cell lineages, forming enteroids (22). The role and importance of Dclk1 as an epithelial normal stem cell marker, in mice, thus continues to evolve and change.
The role of DCLK1 in transformed cells of human origin was examined using isogenic clones of human embryonic epithelial cells (HEK293) (23), which either expressed the empty vector (HEKC) or expressed the full-length progastrin (PG) peptide1-80 (HEKmGAS). PG peptide induces hyper proliferation of colonic crypts and increases colon-carcinogenesis by many fold, in response to AOM±DSS (19,20,24-27). Besides, PG is expressed by >80% of human colon cancer cell lines (hCCCs) and CRCs (28,29). Surprisingly, overexpression of PG, resulted in imparting tumorigenic/metastatic phenotype to HEK293 cells, associated with significant up-regulation of DCLK1+ cell populations in HEKmGAS vs. HEKC cells (23). Down-regulation of DCLK1 expression in HEKmGAS cells caused loss of tumorigenic/metastatic potential of the cells, which led us to conclude that DCLK1 is required for sustaining tumorigenic/metastatic potential of transformed HEKmGAS cells (23), and likely marks both normal stem cells (NSCs) and cancer stem cells (CSCs) in humans. Experiments with mouse models of tumorogenesis have confirmed a critical role of Dclk1 in tumorigenesis, and as a CSC marker, as described below. It is important to note that overexpression of PG in either mature colonic epithelium (as in Fabp-PG mice) (19,24-26) or in intestinal epithelial (IEC) cells (30), results in hyper proliferation of colonic crypts/cells, but does not cause neoplastic transformation (IEC-cells) or formation of colonic tumors (Fabp-PG mice) in the absence of AOM±DSS (19,24-26,30). However, overexpression of PG in embryonic epithelial cells, allowed neoplastic transformation of the epithelial cells confirming hyper-sensitivity of multi-potent embryonic stem cells, unlike mature stem cells. A significant finding was the fact that CSCs in HEKmGAS cells, co-expressed several stem cell markers, along with DCLK1, such as CD44 and Lgr5 (23), while normal cells (NSCs?) in mouse (19) and human colons (unpublished data from our laboratory) were positive for either only DCLK1 or Lgr5 or CD44. In hCCCs, we similarly reported co-expression of DCLK1 with CD44 (31). Dclk1+NSCs, from mouse colons, form enteroids, positive for all differentiated lineages (22). Embryonic hNSCs (in HEK293 cells) formed well-rounded spheroids in vitro (23); presence of differentiated lineages was not examined. The transformed CSCs from HEKmGAS and hCCCs, on the other hand, formed ill-defined spheroids with amorphous structures, which correlated with the metastatic potential of the cells (23,31). Thus morphologically, the nature of spheroidal growths may reflect metastatic potential of CSCs in tumorous growths, which needs further investigation.
It is by now well known that CSCs are resistant to radiation/chemotherapy (32), and targeting CSCs, while sparing NSCs, has remained a challenge in the cancer field (32). We recently reported that a subset of DCLK1 + CSCs are resistant to inhibitory effects of potent chemopreventive agents such as curcumin (31), which may explain the failure of curcumin in clinical trials with cancer patients. Even though curcumin and several chemopreventive agents have been reported to attenuate multiple inflammatory and oncogenic pathways [discussed in (30,31)], for reasons unknown, a subset of DCLK1+ colon cancer cells, survive inhibitory effects of chemopreventive/chemotherapeutic agents by undergoing autophagy, associated with survival rather than apoptosis (31). Down-regulation of DCLK1 was required in combination with chemopreventive agents for eliminating CSCs and avoiding relapse (in terms of reformation of tumorospheres from colon cancer cells) (31). Thus reports in literature to-date suggest a critical role of DCLK1 in maintaining the growth of human colon cancer cells in vitro and in vivo.
Dclk1+ cells, including tuft cells, have similarly been reported to be critically required for colon/pancreatic tumorigenesis in elegantly designed mutant mouse models (33-36). Reporter genes and diphtheria toxin gene were expressed downstream of 5’ promoter of the mDclk1 gene, using either the bac construct (to avoid disruption of endogenous Dclk1 gene) (34-36), or in a mono-allelic manner (33) in mutant mouse models of tumorigenesis. Based on the results, the authors concluded that Dclk1 represented a specific marker of CSCs, and that Dclk1 was required for colon/pancreatic tumorigenesis in mice (33-36). Thus both the mouse and human studies clearly implicate a critical role of DCLK1 in colon carcinogenesis and in the maintenance of tumorous growths. Since neuronal cells and several normal epithelial cells (NSCs?) also express DCLK1, targeting DCLK1 expression, transcribed from the 5’ promoter, can potentially prove to be toxic for many neuronal functions in the adult brain, such as neuronal migration/cognitive behavior, and potentially affect the functions of DCLK1 in normal epithelial cells.
Epigenetic regulation of hDCLK1 gene in CRCs was first described in 2014 (37). Vedeld et al. (37) reported hypermethylation of CpG island in the promoter of hDCLK1 gene in ~82% of hCRC samples, with minimal methylation of the 5’promoter in normal mucosal samples. The authors used three primer sets along the length of the cDNA sequence for hDCLK1-transcript, and amplified sequences corresponding to exons 3/4, 5/6 and 10/12 by RT-PCR. A significant correlation was found between hypermethylation of 5’ promoter and loss of transcripts from exons 3/4 and 5/6, but not exons 10/12 (37). These results provided the first indication that hypermethylation was associated with loss of expression of a significant portion of DCLK1 transcript in hCRC samples (37). The authors analyzed 74 cancer cell lines derived from 15 different issues, and once again measured a significant correlation between promoter methylation of hDCLK1 gene and loss of amplification of transcripts from specific exons, as described above (37). The authors have since patented hypermethylation of hDCLK1 gene promoter as a novel epigenetic biomarker for CRCs in patients. Since then, 5’promoter of hDCLK1 gene has been reported to be hypermethylated in gastric (38), pancreatic (38), and lung cancers (39). Interpretation of data in light of these important discoveries, has however remained ignored by some investigators, who have published since 2014 in this field (36,40). A link to our comments, regarding inaccurate interpretation of some data published by Westphalen et al. (36), is available at http://www.cell.com/cell-stem-cell/comments/S1934-5909(16)30003-0.
While recent literature demonstrates hypermethylation and epigenetic silencing of 5’ promoter of hDCLK1 gene in several human cancers, we and others have reported high levels of DCLK1 protein in hCRCs and human colon cancer cell lines (hCCCs) (23,31,41-44). High levels of DCLK1 staining in hCRC samples was reported to be associated with an increase in cancer specific mortality (43), suggesting possible prognostic value of measuring expression levels of DCLK1 in CRCs. We began examining possible use of an alternate promoter for expression of DCLK1 protein, to resolve the discrepancy between silencing of 5’promoter and presence of DCLK1 in hCRCs/hCCCs. After extensive literature search and in silico analysis of hDCLK1 gene, we discovered that the previously suggested β promoter (11,45), was located in IntronV of the hDCLK1 gene, and had a canonical TATA box (9). The alternate β promoter was transcriptionally active in 80-90% of hCCCs and transformed (HEKmGAS) cells, but was inactive in non-transformed (HEK293) and normal colonic epithelial cells (9). A transcript originating from the alternate β promoter was confirmed to match isoform 2 in the NCBI data base (NM_001195415.1) (9). The full length transcript originating from the 5’ promoter is labeled as isoform 1 (NM_004734.4) in the NCBI data base. Unique primer sets which amplified transcripts from isoforms 1 or 2 were designed (9), and the expression of full length isoform 1 (~82 KDa) was confirmed in normal colonic mucosal samples of patients, while the shorter isoform 2 (47 KDa) was expressed at variable levels in colonic adenomas and adenocarcinomas from patients, at both RNA and protein levels (9). The use of α/β promoters for expressing L or S isoforms of DCLK1 by normal vs. cancer cells is diagrammatically shown in Figure 1.
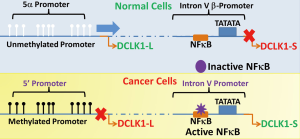
In mice, on the other hand, the full length isoform 1 continues to be expressed by mouse epithelial tumors as suggested from studies with mouse models of colon and pancreatic tumorigenesis (33-36), and as confirmed by us (9). Thus, unlike humans, the 5’promoter of mDclk1 gene does not get hypermethylated and silenced during colon carcinogenesis in response to carcinogens or loss of APC function (34-36). In recent publications, however, this important difference in the isoform(s) being expressed by colonic tumors in humans vs. mouse models has been ignored (36,40), requiring re-evaluation of results reported in these and other articles, published previously. The various mouse and human isoforms of DCLK1 have been given many different names in literature, leading to much confusion in this field, as discussed (9). Even though isoform 2 bears >98% homology with isoform 1, isoform 2 is significantly smaller and lacks the N-terminal double-cortin domains, which can potentially result in significant differences in the 3D structure of the two isoforms. The crystal structure of the full-length isoforms 1 and 2 remains unknown to-date, even though the crystal structure of specific domains has been published, as described above. In a preliminary study we recently reported that the biological activity of DCLK1-S (isoform 2) was significantly different from that of DCLK1-L (isoform 1) (46); hCCCs expressing DCLK1-S developed an invasive phenotype while hCCCs expressing neither or overexpressing isoform 1 alone, lacked invasive and metastatic potential (46). It is therefore speculated that human epithelial cancers, including hCRCs, positive for DCLK1-S expressing CSCs, will form metastatic lesions within shorter intervals, compared to cancerous tumors negative for DCLK1-S expressing CSCs. The latter possibility is supported by results from a cohort of 92 CRC patients (9); high expressers of DCLK1-S in their tumor samples had worse overall survival/disease-free interval, than low expressers, irrespective of stage of disease. On the other hand, relative expression of DCLK1-L in colonic adenocarcinomas of CRC patients did not correlate with survival of the patients (unpublished data from our laboratory). While the epithelial component of CRC tumors mainly expresses DCLK1-S, tumor stromal cells mainly express the L isoform, which may explain an absence of significant correlation between relative expression of L isoform and overall survival of the patients.
A possible important role of DCLK1-L, at early stages of colon carcinogenesis, however, cannot be ignored, given that tissue specific loss of Dclk1-L expression results in the absence of colon/pancreatic tumorigenesis in mouse models of cancer (33-36). Thus, CSCs expressing DCLK1-L may similarly play an important role during tumorigenesis in humans as well. However, it is also true that in mouse models of colon carcinogenesis, metastatic lesions to the liver or lung have not been reported. It is therefore speculated that lack of expression of DCLK1-S by mouse tumors, may render the tumors non-invasive/non-metastatic, unlike hCRCs, based on the results of our preliminary findings (46). There may thus be significant differences in the biology of epithelial tumors expressing long ± short isoforms of DCLK1, which needs to be further explored. Due to significant differences in the epigenetic regulation of DCLK1 in mice and humans during tumorigenesis of epithelial cells, mouse models may need to be developed which mimic the epigenetics of human cancers in order to use mice for pre-clinical validation of preventive/therapeutic agents, and for understanding cellular mechanisms of tumorigenesis. The nude mouse model has many advantages since it allows the growth of human cancer cells, but does not replicate micro-environment of human tumors, and is not useful for examining various stages of tumorigenesis. There is thus a critical need for developing better in vivo models in the cancer field.
Acknowledgements
Funding: This work was supported by RO1 grant (CA97959) from the NCI to P Singh.
Footnote
Provenance: This is a Guest Perspective commissioned by Editor-in-Chief Zhizhuang Joe Zhao (Pathology Graduate Program, University of Oklahoma Health Sciences Center, Oklahoma City, USA).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Omori Y, Suzuki M, Ozaki K, et al. Expression and chromosomal localization of KIAA0369, a putative kinase structurally related to Doublecortin. J Hum Genet 1998;43:169-77. [Crossref] [PubMed]
- Silverman MA, Benard O, Jaaro H, et al. CPG16, a novel protein serine/threonine kinase downstream of cAMP-dependent protein kinase. J Biol Chem 1999;274:2631-36. [Crossref] [PubMed]
- Matsumoto N, Pilz DT, Ledbetter DH. Genomic structure, chromosomal mapping, and expression pattern of human DCAMKL1 (KIAA0369), a homologue of DCX (XLIS). Genomics 1999;56:179-83. [Crossref] [PubMed]
- Burgess HA, Martinez S, Reiner O. KIAA0369, doublecortin-like kinase, is expressed during brain development. J Neurosci Res 1999;58:567-75. [Crossref] [PubMed]
- Tuy FP, Saillour Y, Kappeler C, et al. Alternative transcripts of Dclk1 and Dclk2 and their expression in doublecortin knockout mice. Dev Neurosci 2008;30:171-86. [PubMed]
- Burgess HA, Reiner O. Doublecortin-like kinase is associated with microtubules in neuronal growth cones. Mol Cell Neurosci 2000;16:529-41. [Crossref] [PubMed]
- Lin PT, Gleeson JG, Corbo JC, et al. DCAMKL1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization. J Neurosci 2000;20:9152-61. [PubMed]
- Burgess HA, Reiner O. Cleavage of doublecortin-like kinase by calpain releases an active kinase fragment from a microtubule anchorage domain. J Biol Chem 2001;276:36397-403. [Crossref] [PubMed]
- O'Connell MR, Sarkar S, Luthra GK, et al. Epigenetic changes and alternate promoter usage by human colon cancers for expressing DCLK1-isoforms: Clinical Implications. Sci Rep 2015;5:14983. [Crossref] [PubMed]
- Kim MH, Derewenda U, Devedjiev Y, et al. Purification and crystallization of the N-terminal domain from the human doublecortin-like kinase. Acta Crystallogr D Biol Crystallogr 2003;59:502-5. [Crossref] [PubMed]
- Shang L, Kwon YG, Nandy S, et al. Catalytic and regulatory domains of doublecortin kinase-1. Biochemistry 2003;42:2185-94. [Crossref] [PubMed]
- Shu T, Tseng HC, Sapir T, et al. Doublecortin-like kinase controls neurogenesis by regulating mitotic spindles and M phase progression. Neuron 2006;49:25-39. [Crossref] [PubMed]
- Shin E, Kashiwagi Y, Kuriu T, et al. Doublecortin-like kinase enhances dendritic remodelling and negatively regulates synapse maturation. Nat Commun 2013;4:1440. [Crossref] [PubMed]
- Le Hellard S, Håvik B, Espeseth T, et al. Variants in doublecortin- and calmodulin kinase like 1, a gene up-regulated by BDNF, are associated with memory and general cognitive abilities. PLoS One 2009;4:e7534. [Crossref] [PubMed]
- Schenk GJ, Veldhuisen B, Wedemeier O, et al. Over-expression of δC-DCLK-short in mouse brain results in a more anxious behavioral phenotype. Physiol Behav 2010;101:541-8. [Crossref] [PubMed]
- Giannakis M, Stappenbeck TS, Mills JC, et al. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem 2006;281:11292-300. [Crossref] [PubMed]
- May R, Riehl TE, Hunt C, et al. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 2008;26:630-7. [Crossref] [PubMed]
- May R, Sureban SM, Hoang N, et al. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells 2009;27:2571-9. [Crossref] [PubMed]
- Sarkar S, Swiercz R, Kantara C, et al. Annexin A2 mediates up-regulation of NF-κB, β-catenin, and stem cell in response to progastrin in mice and HEK-293 cells. Gastroenterology 2011;140:583-595.e4. [Crossref] [PubMed]
- Jin G, Ramanathan V, Quante M, et al. Inactivating cholecystokinin-2 receptor inhibits progastrin-dependent colonic crypt fission, proliferation, and colorectal cancer in mice. J Clin Invest 2009;119:2691-701. [PubMed]
- Gerbe F, Brulin B, Makrini L, et al. DCAMKL-1 expression identifies Tuft cells rather than stem cells in the adult mouse intestinal epithelium. Gastroenterology 2009;137:2179-80; author reply 2180-1. [Crossref] [PubMed]
- Chandrakesan P, May R, Qu D, et al. Dclk1+ small intestinal epithelial tuft cells display the hallmarks of quiescence and self-renewal. Oncotarget 2015;6:30876-86. [PubMed]
- Sarkar S, Kantara C, Ortiz I, et al. Progastrin overexpression imparts tumorigenic/metastatic potential to embryonic epithelial cells: phenotypic differences between transformed and nontransformed stem cells. Int J Cancer 2012;131:E1088-99. [Crossref] [PubMed]
- Singh P, Velasco M, Given R, et al. Progastrin expression predisposes mice to colon carcinomas and adenomas in response to a chemical carcinogen. Gastroenterology 2000;119:162-71. [Crossref] [PubMed]
- Cobb S, Wood T, Ceci J, et al. Intestinal expression of mutant and wild-type progastrin significantly increases colon carcinogenesis in response to azoxymethane in transgenic mice. Cancer 2004;100:1311-23. [Crossref] [PubMed]
- Umar S, Sarkar S, Cowey S, et al. Activation of NF-kappaB is required for mediating proliferative and antiapoptotic effects of progastrin on proximal colonic crypts of mice, in vivo. Oncogene 2008;27:5599-611. [Crossref] [PubMed]
- Umar S, Sarkar S, Wang Y, et al. Functional cross-talk between beta-catenin and NFkappaB signaling pathways in colonic crypts of mice in response to progastrin. J Biol Chem 2009;284:22274-84. [Crossref] [PubMed]
- Singh P, Owlia A, Varro A, et al. Gastrin gene expression is required for the proliferation and tumorigenicity of human colon cancer cells. Cancer Res 1996;56:4111-5. [PubMed]
- Singh P, Sarkar S, Kantara C, et al. Progastrin Peptides Increase the Risk of Developing Colonic Tumors: Impact on Colonic Stem Cells. Curr Colorectal Cancer Rep 2012;8:277-89. [Crossref] [PubMed]
- Singh P, Sarkar S, Umar S, et al. Insulin-like growth factors are more effective than progastrin in reversing proapoptotic effects of curcumin: critical role of p38MAPK. Am J Physiol Gastrointest Liver Physiol 2010;298:G551-62. [Crossref] [PubMed]
- Kantara C, O'Connell M, Sarkar S, et al. Curcumin promotes autophagic survival of a subset of colon cancer stem cells, which are ablated by DCLK1-siRNA. Cancer Res 2014;74:2487-98. [Crossref] [PubMed]
- Wang T, Shigdar S, Gantier MP, et al. Cancer stem cell targeted therapy: progress amid controversies. Oncotarget 2015;6:44191-206. [PubMed]
- Nakanishi Y, Seno H, Fukuoka A, et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet 2013;45:98-103. [Crossref] [PubMed]
- Westphalen CB, Asfaha S, Hayakawa Y, et al. Long-lived intestinal tuft cells serve as colon cancer-initiating cells. J Clin Invest 2014;124:1283-95. [Crossref] [PubMed]
- Bailey JM, Alsina J, Rasheed ZA, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology 2014;146:245-56. [Crossref] [PubMed]
- Westphalen CB, Takemoto Y, Tanaka T, et al. Dclk1 Defines Quiescent Pancreatic Progenitors that Promote Injury-Induced Regeneration and Tumorigenesis. Cell Stem Cell 2016;18:441-55. [Crossref] [PubMed]
- Vedeld HM, Skotheim RI, Lothe RA, et al. The recently suggested intestinal cancer stem cell marker DCLK1 is an epigenetic biomarker for colorectal cancer. Epigenetics 2014;9:346-50. [Crossref] [PubMed]
- Vedeld HM, Andresen K, Eilertsen IA, et al. The novel colorectal cancer biomarkers CDO1, ZSCAN18 and ZNF331 are frequently methylated across gastrointestinal cancers. Int J Cancer 2015;136:844-53. [Crossref] [PubMed]
- Powrózek T, Krawczyk P, Nicoś M, et al. Methylation of the DCLK1 promoter region in circulating free DNA and its prognostic value in lung cancer patients. Clin Transl Oncol 2016;18:398-404. [Crossref] [PubMed]
- Weygant N, Ge Y, Qu D, et al. Survival of Patients with Gastrointestinal Cancers Can Be Predicted by a Surrogate microRNA Signature for Cancer Stem-like Cells Marked by DCLK1 Kinase. Cancer Res 2016. [Epub ahead of print].
- Sureban SM, May R, Ramalingam S, et al. Selective blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a MicroRNA-dependent mechanism. Gastroenterology 2009;137:649-59, 659.e1-2.
- Kantara C, O'Connell MR, Luthra G, et al. Methods for detecting circulating cancer stem cells (CCSCs) as a novel approach for diagnosis of colon cancer relapse/metastasis. Lab Invest 2015;95:100-12. [Crossref] [PubMed]
- Gagliardi G, Goswami M, Passera R, et al. DCLK1 immunoreactivity in colorectal neoplasia. Clin Exp Gastroenterol 2012;5:35-42. [Crossref] [PubMed]
- Gagliardi G, Moroz K, Bellows CF. Immunolocalization of DCAMKL-1, a putative intestinal stem cell marker, in normal colonic tissue. Pathol Res Pract 2012;208:475-9. [Crossref] [PubMed]
- Pal S, Gupta R, Kim H, et al. Alternative transcription exceeds alternative splicing in generating the transcriptome diversity of cerebellar development. Genome Res 2011;21:1260-72. [Crossref] [PubMed]
- O’Connell MR, Sarkar S, Ward D, et al. Short (S) Isoform of Cancer-Stem-Cell Marker, DCLK1, Is Critically Required for Maintaining Proliferative/Tumorigenic Potential of Human Colon Cancer Cells (hCCCs) Independent of DCLK1-L Isoform. Gastroenterology 2016;150:S605.
Cite this article as: Singh P, O’Connell M, Shubhashish S. Epigenetic regulation of human DCLK-1 gene during colon-carcinogenesis: clinical and mechanistic implications. Stem Cell Investig 2016;3:51.


