New hope for chronic myelogenous leukemia patients: dasatinib offers better efficacy with shorter treatment
Chronic myelogenous leukemia (CML) is a clonal myeloproliferative neoplasm that is caused by generation of a BCR-ABL1 fusion gene encoding a constitutively active tyrosine kinase (1). The discovery of small molecule tyrosine kinase inhibitors (TKIs) targeting ABL1 tyrosine kinase, such as the first-generation TKI imatinib mesylate (IM) and the second-generation TKIs dasatinib, nilotinib and bosutinib, has dramatically improved the prognoses of CML patients (1,2). However, long-term treatment with TKIs does not appear to completely cure most CML patients (3-9), and the majority continues on these therapies for fear of suffering a relapse if they stop their treatment. Thus, it has become important to provide CML patients with solid information and some hope that they can eventually safely discontinue or finish their TKI therapy regimens, which have side-effects and are expensive.
The “Stop IM” (STIM) trial conducted by Mahon et al. investigated if and how treated CML patients could maintain a cure after stopping TKI therapy. Importantly, the results of this ground-breaking effort indicated that 39% of CML patients who underwent IM treatment and had achieved deep molecular response (DMR) for at least 2 years were effectively cured of their CML disease and could safely stop the TKI therapy (10). Unfortunately, the remaining 61% of treated patients suffered relapses of CML disease after IM was discontinued (10). Thus, many hematologists wondered whether or not CML recurrence could be further reduced by treatment with the second-generation TKI dasatinib after the therapy was stopped. Some benefit was expected because dasatinib has more extensive inhibitory activity than IM (11-13). In the paper that is the subject of this Perspective, Imagawa et al. report on the safety and efficacy of a stop study of dasatinib therapy in a multicenter phase II trial (UMIN000005130), termed the “dasatinib discontinuation” (DADI) trial (14). This trial enrolled CML patients who had already received IM therapy and then had changed to second-line or subsequent dasatinib therapy. The objective was to carefully evaluate whether or not CML disease would relapse after stopping the dasatinib therapy; in other words, could CML patients be cured by dasatinib treatment and maintain that cure in the absence of the drug. Rather than the 2-year DMR period in the STIM trial (10), the DADI trial examined benefits for CML patients who maintained a 1-year period of DMR following dasatinib treatment. Importantly, of the 63 patients who discontinued dasatinib treatment after sustaining DMR for at least 1 year, 30 patients (48%) were able to successfully sustain DMR for another 12 months (14). Even though the remaining 33 of the 63 patients (52%) suffered a relapse of CML disease, rapid molecular responses were confirmed in all 33 of these patients after dasatinib therapy was restarted. Imagawa et al. concluded that a substantial proportion of CML patients who receive dasatinib treatment and show DMR for 1 year may be potential candidates for treatment discontinuation (14).
Dasatinib interacts with both the active and inactive conformations of the ABL1 tyrosine kinase, whereas IM binds only to the catalytically inactive form (15). Although both TKIs can inhibit ABL1 kinase functions, the structural differences between these drugs may allow dasatinib to exert inhibitory activity on a broad spectrum of kinases (15). Indeed, dasatinib inhibits not only ABL1 kinase, but also cKIT, PDGF, ephrin receptor kinase, SRC, and the Src family kinases FGR, FYN, HCK, LCK and YES (15). Importantly, dasatinib may also be implicated in suppressing signal transduction in cells on the immune system.
Previous studies have indicated that the CML patients who have the most favorable prognoses following dasatinib therapy are those bearing a relatively high number of large granular lymphocytes (16-18). In the STIM trial, the CML patients with the best prognoses after cessation of IM therapy had an increased number of natural killer (NK) cells (10). In same vein, Imagawa et al. found that a high frequency of NK cells was associated with improved treatment-free survival of CML patients after DADI (14). The molecular mechanism by which dasatinib might regulate NK cell survival, and how high numbers of these NK cells contribute to the suppression of CML disease relapse, are mysteries. Nevertheless, these results suggest that NK cell-based immune surveillance may play an important role (Figure 1).
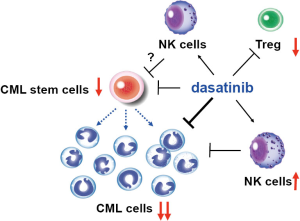
It is now widely accepted that CML is a stem cell disorder whose cell-of-origin is the normal hematopoietic stem cells (HSCs) (1). It has recently been shown that quiescent CML stem cells are insensitive to TKI therapy, and that reproliferation of these residual CML stem cells is responsible for CML recurrence following TKI therapy (19). Interestingly, administration of dasatinib alone can eliminate the CML stem cell population in a murine CML model (20). It remains unclear whether dasatinib directly eradicates CML stem cells through an off-target effect, or whether dasatinib’s enhancement of the NK cell population supports an innate immune response that contributes to CML stem cell eradication; indeed, both mechanisms may be involved. In addition, IM treatment has been shown to deplete the highly suppressive FoxP3+ regulatory T (Treg) cells in CML patients, thereby allowing anti-cancer immune responses to proceed fully and deliver a therapeutic effect (21). Dasatinib can also suppress Treg cells (22), although the molecular mechanism by which TKIs might inhibit a kinase activity responsible for controlling Treg functions is elusive. Thus, further investigations of the effects of dasatinib on immune suppression/tolerance and/or the self-renewal capacity of CML stem cells may yield tangible contributions to the early diagnosis of disease recurrence in CML patients. Moreover, a combination of dasatinib plus a novel agent supporting CML immunotherapy or CML stem cell therapy may constitute a new approach to preventing CML relapses in the future. Of note, a new version of a DADI trial (UMIN000011099) is under way for CML patients who have received at least 3 years of first-line dasatinib therapy and have maintained DMR for at least the last 12 months. Based on the work of Imagawa et al., a large proportion of patients in this trial can expect a significant curative benefit even after they stop their TKI therapy. The future looks bright for advances in CML TKI-based treatments.
Acknowledgements
The authors are grateful to Kumi Oshima for her great support and helpful discussions.
Funding: Supported by in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI (No. 26290038).
Footnote
Conflicts of Interest: K Naka received a research grant from Carna Bioscience, Inc.; T Ichinohe has no conflicts of interest to declare.
References
- Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer 2005;5:172-83. [Crossref] [PubMed]
- O'Hare T, Zabriskie MS, Eiring AM, et al. Pushing the limits of targeted therapy in chronic myeloid leukaemia. Nat Rev Cancer 2012;12:513-26. [Crossref] [PubMed]
- Bhatia R, Holtz M, Niu N, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood 2003;101:4701-7. [Crossref] [PubMed]
- Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 2006;355:2408-17. [Crossref] [PubMed]
- Hochhaus A, O'Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia 2009;23:1054-61. [Crossref] [PubMed]
- Giles FJ, Rosti G, Beris P, et al. Nilotinib is superior to imatinib as first-line therapy of chronic myeloid leukemia: the ENESTnd study. Expert Rev Hematol 2010;3:665-73. [Crossref] [PubMed]
- Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010;362:2260-70. [Crossref] [PubMed]
- Ross DM, Branford S, Seymour JF, et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia 2010;24:1719-24. [Crossref] [PubMed]
- Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood 2011;117:3733-6. [Crossref] [PubMed]
- Mahon FX, Réa D, Guilhot J, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol 2010;11:1029-35. [Crossref] [PubMed]
- McCormack PL, Keam SJ. Spotlight on dasatinib in chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. BioDrugs 2012;26:61-4. [Crossref] [PubMed]
- Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood 2007;109:2303-9. [Crossref] [PubMed]
- Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014;123:494-500. [Crossref] [PubMed]
- Imagawa J, Tanaka H, Okada M, et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): a multicentre phase 2 trial. Lancet Haematol 2015;2:e528-35. [Crossref] [PubMed]
- Weisberg E, Manley PW, Cowan-Jacob SW, et al. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer 2007;7:345-56. [Crossref] [PubMed]
- Kim DH, Kamel-Reid S, Chang H, et al. Natural killer or natural killer/T cell lineage large granular lymphocytosis associated with dasatinib therapy for Philadelphia chromosome positive leukemia. Haematologica 2009;94:135-9. [Crossref] [PubMed]
- Mustjoki S, Ekblom M, Arstila TP, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia 2009;23:1398-405. [Crossref] [PubMed]
- Kreutzman A, Juvonen V, Kairisto V, et al. Mono/oligoclonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood 2010;116:772-82. [Crossref] [PubMed]
- Sinclair A, Latif AL, Holyoake TL. Targeting survival pathways in chronic myeloid leukaemia stem cells. Br J Pharmacol 2013;169:1693-707. [Crossref] [PubMed]
- Naka K, Ishihara K, Jomen Y, et al. Novel oral transforming growth factor-β signaling inhibitor EW-7197 eradicates CML-initiating cells. Cancer Sci 2016;107:140-8. [Crossref] [PubMed]
- Noguchi S, Nishikawa H, Saitoh H, et al. Tyrosine kinase inhibitor imatinib enhances tumor immunity by depleting functionally mature regulatory T cells. Blood 2015;126:2219.
- Fei F, Yu Y, Schmitt A, et al. Dasatinib inhibits the proliferation and function of CD4+CD25+ regulatory T cells. Br J Haematol 2009;144:195-205. [Crossref] [PubMed]
Cite this article as: Naka K, Ichinohe T. New hope for chronic myelogenous leukemia patients: dasatinib offers better efficacy with shorter treatment. Stem Cell Investig 2016;3:19.


