Platelet lysate for COVID-19 pneumonia—a newer adjunctive therapeutic avenue
Introduction
It is astounding to know that human blood serves as a source for many therapeutic products. These therapeutic products can be classified into cellular and protein-based blood products. The cellular blood products include red blood cells, buffy coat derived, and apheresis platelets, whereas protein-based products are fresh frozen plasma, cryoprecipitate, and plasma fractionation products. Some products of blood are being recognized as “essential medicines” by the World Health Organisation, emphasizing their vital significance in any national healthcare system (1).
Although anuclear platelets are well recognized for their crucial role in hemostasis, global researchers have proved numerous non-hemostatic immunologic functions of platelets in vertebrates. A definitive complex cross-talk exists between their hemostatic and non-hemostatic functions. Platelets naturally release their growth factors in a gradual and targeted manner to enhance healing. However, at times a physician might need to advocate platelets containing growth factors to an area for its prompt recovery (2). Currently, there are means available to lyse platelets using an optimal methodology to render the myriads of growth factors accessible to the body.
A newer blood product preparation called human platelet lysate (HPL) has been developed recently (3). Notably, being rich in platelet growth factors makes it an ideal candidate for utilization as a clinical-grade, xeno-free, supplement of growth media for human cell propagation and cell therapy procedures (3,4). In this review article, the rational usage of platelet lysate to combat COVID-19 has been discussed, and the need for undertaking prospective randomized controlled trials to establish its due efficacy and safety has been equally emphasized in conjunction with the prevailing pandemic scenario.
Materials and methods
A scoping review was done till May 2020 from PubMed, Google Scholar, Scopus, PubMed Central, and Medline databases using the following search terms namely, platelet lysate, COVID-19, growth factors, ARDS and Influenza. Two independent reviewers collected 123 articles and after rapid scanning of the titles, a total of 72 articles were finally chosen for the review. A framework was developed for the analysis of the articles, following which the various diseases were classified based on the mode of action of platelets and platelet lysate in them.
Results
A scoping review showed several plausible uses of platelets and platelet lysate on various systemic diseases and viral infections.
Platelets
Structure of Platelets
Platelets are anucleated cells having a biconvex discoid shape that measures 2 to 3 microns in their greatest diameter (5). The circulating cytoplasmic fragments, called platelets, are derived from megakaryocytic progenitor cells of bone marrow. The ratio of platelets to red blood cells in a healthy adult ranges from 1:10 to 1:20 (5).
Platelet kinetics
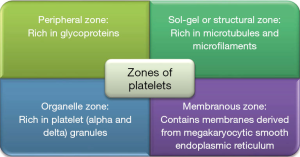
Thrombopoietin is a hormone produced by the kidneys and liver which induces platelet production. During its entire life span, each megakaryocyte produces platelets ranging from 1,000 to 3,000. The daily production is approximately 1011 platelets. The average life span of circulating platelets is around 8–9 days. One unit of platelet concentrate increases the platelet count by 5,000–8,000/mm3. They are regulated by internal apoptotic Bcl-xL genes. The site of platelet reserve is the spleen and the senescent platelets undergo phagocytosis in the spleen and liver. The zones of platelet have been explained in Figure 1 (6).
Platelet lysate: properties and mechanism
Despite being anuclear, platelets are the mega reservoir of growth factors and are activated at sites of endothelial and tissue injury. Hence, these pave way for tissue repair, regeneration, and revascularization of the injured tissue (7).
The genesis of platelet biology and its contents are depicted in Figure 2. Platelets possess various granules (alpha, delta, gamma, and lambda), lysosomes, and microparticles (growth factors, pro, and antiangiogenic factors) in their intracellular content. The regulatory molecules produced by platelets upon activation are angiopoietin-1, vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), hepatocyte growth factor (HGF), insulin-like growth factor 1 (IGF-1), brain-derived neurotrophic factor (NGF), basic fibroblast growth factor (FGF-b), connective tissue growth factor and thrombospondin respectively (2). Besides, coagulation factors and anticoagulants are also their key contents.
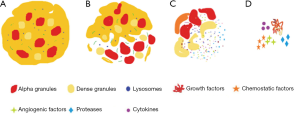
To add on, anti-microbial factors (complement precursors C3 and C4, complement factor D, matrix metalloproteinases), proteases such as thymosin B2, cathepsin D, and E, C1 inhibitor, and/or substances for the surrounding cells, such as adenosine diphosphate (ADP), adenosine triphosphate (ATP), histamine, serotonin, adrenaline and a variety of chemokine factors (IL-1Ra, IL-6, IL-4, IL-1) account for the regenerative role of platelets. Being a warehouse of these factors, platelets play a pivotal role in hemostasis inflammation, angiogenesis, and regeneration (8). And that’s how platelets emerge as key players; blessed with the capacity to alter hemodynamic properties.
Platelet lysate is constituted by acellular formulation having platelet proteins. It is obtained by lysing the platelet plasma membrane. During its processing, centrifugation and filtration techniques facilitate the removal of all cellular debris and yield content generally rich in growth factors. The white cell antigens are low, which minimizes the risk of adverse immune responses. In the connotation of its regenerative role, the myriads of the growth factors render an increase in cell proliferation and angiogenesis. To reiterate, it accounts for the release of a supraphysiological dose of platelet factors (2-4).
It can be obtained via changes in temperature, mechanical lysis, ultrasound bath, lyophilization, repeated freeze-thaw-freeze cycles, and filtration techniques (9). Current studies have shown its utility as a supplement to the culture medium as a replacement for fetal bovine serum (FBS). It has been put forth in vitro studies for the evaluation of the immune-regulatory potential of the lysate. Growth factors can be extracted after lysing the platelets. Notably, it renders a less expensive and safer alternative to recombinant or animal products (10).
The extracellular matrix in platelet-rich plasma (PRP) provides a biodegradable scaffold. The polymerization of fibrinogen into fibrin occurs and subsequently binds to scaffolding proteins such as fibronectin or vitronectin (11). These act as adhesives to collagen and other ECM components facilitating the process of adhesion at injury sites. Additionally, they contain integrin-binding sites (e.g., RGD motifs) which further allow attachment of cells. Platelet-derived scaffolds are degradable by proteases [plasmin and matrix metalloproteinases (MMPs)] and hence, able to achieve full biodegradability and host integration (12).
Platelet immunology
Innate immunity
Platelets possess antimicrobial (via antimicrobial peptides) and phagocytic activity which is possible because of the cross-talks with a wide range of microbial spectra (13,14) and possesses pro-inflammatory cytokines (IL-1α) which modulate inflammatory and immune responses (15). Platelets execute the innate immune mechanism due to the expression of toll-like receptors (TLRs), such as TLR- 2, -4, and -9 which is the basis for platelet-neutrophil interactions and it results in extracellular entrapment that kills the bacteria (16). The thrombotic nature of platelets leads to the containment of invasive microorganisms. As platelets possess these pro-inflammatory cytokines, they form a part of the innate immune system.
Platelets have anti-inflammatory cytokine pockets. Among those anti-inflammatory cytokines, TGF-β is a potent immunosuppressive factor, that downregulates the expression of the activating receptor, natural killer (NK) cells, and finally inhibits the antitumor activity of NK cells (17). Thus, platelets allow tumor cells to evade the host’s immunosurveillance. Thrombospondin-1 (TSP-1) activates the anti-inflammatory cytokine TGF-β1 and inhibits the phagocytic capacity of macrophages (18). Platelet TLRs justify their active role of sentinel cells by sensing the invasion of foreign microorganisms and counteracting by releasing chemokines. These platelets may also escalate the number of inflammatory cells and depute those at the site of infection. Thus, platelets not only act as proinflammatory cells but also modulate the balance between inflammation and immune responses.
Adaptive immunity
CD154, a member of the TNF superfamily, present on platelets, CD4, CD8, and γT cells, is central to the mobilization of the adaptive immune response. T cell CD154 ligation of CD40 on DC promotes DC maturation with its accompanying elevation of costimulatory/adhesion molecule expression and cytokine production, both of which are associated with enhanced antigen presentation (19-23). CD4 T cell-derived CD154 is known to be necessary for providing the second signal during B lymphocyte activation, and as such is required to induce B cell proliferation, enhance expression of costimulatory and adhesion molecules, and enable isotype switching and germinal center (GC) formation and maturation (24,25).
P selectin (CD62P), which is found in the peripheral zone of platelets plays a crucial role in Th-1 immune response development (26). Activated platelets support immune class switch and augment CD8+ T cell function during viral infections by expressing CD40L on their surface (27). These events can directly affect B-cell differentiation, proliferation, and antibody production (28). Thereby platelets actively participate in adaptive immune response generation.
Interestingly, platelets contain functional TGF-β that is essential for the development of naturally occurring Foxp3+ regulatory T cells (Treg) or T helper 17 cells (Th17) depending on the local cytokine environment (29). However, the role of platelets in modulating the balance of immune tolerance and inflammation caused by microorganisms is not known.
Platelet cross-talks
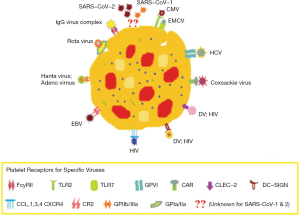
The plausible role of megakaryocytes against invading microbes was not clearly understood. Researchers reported that the beneficial effects in the host were due to the upregulation of immune responses by the interplay between platelets and microbes (30). Various researchers across the globe have documented the interplay between platelet and viruses in in-vitro studies, which resulted in the activation of platelets and lead to virus-laden platelet elimination and further clearance of viral load (31). The receptors for viruses have been depicted in Figure 3.
Platelet activation by either direct or indirect stimuli results in activation, degranulation, and release of pockets of growth factors and biomolecules. The resultant biomolecules mediate host defense mechanisms. A novel biomolecule, kinocidin, which differentiates microbes under different pH conditions, has an immunomodulatory effect through chemotaxis and activation of immune cells (32,33).
The most prominent antiviral platelet kinocidins are platelet factor 4 (PF-4/CXCL4), which is a potent HIV-1 inhibitor, followed by CCL5 and CCL3 (HIV-suppressive factors) (34). Among these chemokines, CCL5 is involved in viral lung infections which are well documented in influenza A virus infection in mice by providing anti-apoptotic signaling for macrophages (35). Platelet–leukocyte-mediated immune responses during viral infections result in enhanced phagocytosis, reactive oxygen species (ROS) production of neutrophils, and neutrophil extracellular trap (NET) formation (36).
The interactions between platelets and monocytes resulted in enhanced monocyte activation and differentiation, boosted expression of tissue factor on the surface of the monocytes, and enhanced the formation of microparticles (37). Platelet–dendritic cell cross-reaction promotes dendritic cell maturation and enhances their antigen-binding capacity (38). Platelet–lymphocyte cross-talk modulates T and B lymphocytes function via direct cell–to–cell interaction and soluble mediators (CCL-5 and CXCL-4 and -7) (39). Chemokines released by platelets (CXCL4 and CCL5) enhance pro-, and anti-inflammatory cytokine production, which are the primary components in T-cell differentiation and further leads to an increase in regulatory T-lymphocytes and limits Th17 differentiation (40,41).
Preparation of platelet lysate for clinical utility
Various researchers followed their protocol in the preparation of platelet lysate and were used for treating various disorders (42-52). Schallmoser et al. described the methodology for the preparation of HPL for clinical utility (10). This includes combining 4–5 platelet concentrates bags having the same-gender characters and storing them at ‒20 °C. Following initial freezing, these are defrosted and subjected to a centrifugation procedure. Afterward, they undergo filtration using sterilizing filters (0.22 μm). This process of filtration facilitates the elimination of membrane debris and minimizes the number of possible antigenic agents. The yielded supernatant will be frozen again at ‒20 °C. This will increase platelet lysis and further release soluble factors. The discussed procedure will be conducted several times until a product free of membrane debris and antigenic factors is obtained. The finally produced HPL will then be divided into 4 mL aliquots. It will be frozen at ‒80 °C until clinically utilized.
Pathophysiology of COVID-19 pneumonia and platelet lysate
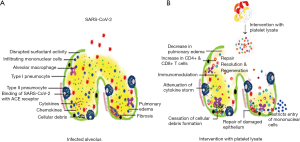
The presence of ACE-2 receptors facilitates the entry of SARS-CoV-2 into the pulmonary alveolar cells. Due to the widespread expression of ACE-2 receptors on pulmonary, cardiac, renal, and hepatic tissues, the patients with severe COVID-19 infection result in a multi-organ dysfunction syndrome complex (53).
The immunopathogenesis in COVID-19 pneumonia ranges from sore throat, fever, dyspnea, anosmia, and dysgeusia to pneumonia which mandates ventilator support. In later stages of diseases, due to the extensive disruption of pulmonary epithelium and alveolar endothelium, an exponential production of cytokines and chemokines results in the development of cytokine storm (54) and hence results in severe acute respiratory distress syndrome (ARDS), systemic inflammatory response syndrome (SIRS), multi-organ dysfunction syndrome (MODS) and eventually death. The pathological findings from the lungs of severe COVID-19 patients showed bilateral diffuse alveolar damage with cellular fibromyxoid exudates (55). Rapid viral replication, cellular damage, and virus-induced ACE2 downregulation are all noted as possible causes of aggressive inflammation caused by SARS-CoV2 (56).
As far as the potential treatment targets are concerned, blockage of IL-6, IL-1, and TNF-α may benefit the patients. Platelet lysate could be a potential therapeutic modality in such cases. The process of generating platelet lysate includes the removal of all cellular debris. This results in a rich content of growth factors with a low concentration of white cell antigens that could trigger immune responses. As it provides supra-physiologic doses of platelet factors (as shown in Figure 4), the platelet lysate might increase cell proliferation and angiogenesis, thus helps in cellular regeneration (3). Platelet lysate has traditionally been studied as a supplement to the culture medium for lymphocytes or as a replacement for FBS. Recently, some studies have focused on the immune regulatory potential of platelet lysate. In one such study, the lysate was able to induce the development of CD4+Foxp3+-induced regulatory T cells (iTregs). These regulatory cells inhibited the proliferation of normal T cells thereby exerting immunosuppressive and anti-inflammatory properties (57). Considering the above evidence, the potential for utilization of platelet lysate (possibly in a nebulized form) as an adjuvant in the treatment of COVID-19 infection-related ARDS can be explored.
Discussion
Platelets are the key blood elements and renowned for their central role in hemostasis. They are a natural reservoir of multiple proteins, cytokines, and growth factors that are stored in a dense membrane system e.g., stored in the α-granules within them. In vivo, platelets are activated at the site of injury whereby they allow the release of a large number of soluble mediators which act as key participants in hemostasis and promoting tissue repair, including revascularization (58,59). The extraction of growth factors through lysis of human platelets represents a safer alternative with decreased costs as compared to the adoption of recombinant growth factors or proteins derived from animals (60). These signaling molecules, when in contact with cell transmembrane receptors promote the expression of genes involved in processes like cellular recruitment, growth, and morphogenesis (61). There are various other molecules present in platelet α-granules which include clotting factors, factors responsible for fibrinolysis, adhesive proteins, proteases, and antiproteases as well as glycoproteins that are constituents of its membrane (62). CD40 ligand and p-selectin molecules involved in inflammation processes are also a component of the platelet α-granules that are released upon platelet activation (63,64). Therefore, if we see therapeutically, the application of platelets seems to be quite promising for regenerative medicine and tissue engineering applications.
For therapeutic applications, platelets are commonly used in the form of PRP wherein the normal concentration of platelets is at least 1×106 platelets/μL in 5 mL of plasma, whereas the range in whole blood is from 1.5×105 to 3.5×105 platelets/μL respectively (65,66). In addition to this, HPL equally embraces possibly suitable utilization in the specialty of regenerative medicine. PL has been suggested as an efficient alternative to FBS for cell and tissue expansion, reducing the risks of transmission of xenogeneic contaminants as virus, bacteria, and prions, as well as xenogeneic antigens. Several ethical issues are associated with the collection methods of animal serums and the potential limits of availability (3,66). Increased safety for cell therapy protocols and cost reduction are the major benefits of human alternatives (67-69).
Cells from marrow, as well as trabecular bone, cells from dental pulp, mesenchymal stem cells (MSCs), adipose tissue-derived stem cells, human articular chondrocytes, myofibroblasts, human immortal keratinocyte cell line, and osteoblasts, respond to the use of HPL with increased proliferation rate when compared to animal-derived mediums or mixtures of recombinant growth factors (67-70). It was also reported that the use of PL in substitution of FBS does not interfere with the differentiation potential and immunophenotypic characteristics, at least in the case of MSCs (70). The proliferation process is highly influenced by the presence of cytokines, growth factors, and attachment factors. These molecules are responsible for cellular migration, redistribution, adhesion, and proliferation and they are present in HPL. HPL contains a total protein content typically exceeding 50–55 mg/mL while FBS composition includes a protein content that is on average 38 mg/mL, this may explain the higher proliferation rates in cells cultured using HPL as a culture media supplement.
Frequently, HPL used as a supplement for cell culture media is obtained from processed blood donations from FDA or EMA licensed blood centers. It is also now possible to obtain commercial sources of HPL to replace FBS respectively. To decrease the variability between different batches, numerous blood donor units are processed together.
Despite the promising results, a major concern regarding the use of Plasma lysate is their heterogeneity and demanding characterization. There is an essential demand for HPL quality control procedures, which may comprise analysis of pivotal substances important for cell growth and differentiation. Advanced characterization techniques such as mass spectrometry and two-dimensional gel electrophoresis and enzyme-linked immunosorbent analyses could be important for better characterization and standardization in the use of HPL (3,71). Testing for infectious markers or pathogen inactivation is also important process in HPL preparation. In the case of infectious markers, each HPL sample should be submitted to the detection analysis of viral markers such as HIV, Hep-B, and Hep-C through nucleic acid amplification methods. Pathogen inactivation during the HPL obtaining process reduces the risk of pathogen transmissions such as viruses, bacteria, fungi, and prions.
Karen Bieback from the University of Mannheim gave an audit on the preclinical evidence for the role of HPL in regenerative functions and cell growth-supporting functions. This was shown by using the expansion of MSCs derived from bone marrow and proposed HPL to be an intermediate growth medium supplement that can be used in place of FBS during further scientific advancements until chemically defined media is available additionally (72).
Since HPL is of biological origin, besides testing for the three viral markers, it also requires further testing to verify its purity, its microbial safety, and the determination of the storage environment needed. As mentioned earlier, to decrease the variability between different batches, numerous blood donor units need to be processed together. Also, bigger pools will tend to upsurge the hazard of transmitting infectious agents. Therefore manufacturers either put a check on the number of donations per pool or arrange for methods for inactivation/removal of viruses.
A major drawback lies in predicting the precise qualitative composition and this finally ends up in the detailed analysis of the composition. In fact, in certain relevant instances, these testing procedures also include the analysis of cell-based impurities. It is significant to note that safety measures are of utmost value while using HPL as a pooled product because of preventing transmission of blood-derived infections. A few reports have mentioned the transmission of Hepatitis E virus and Parvo B19 virus in blood products and highlighted the significance of safety considerations respectively. The recommendations made by the Paul Ehrlich Institute, Germany includes switching to a smaller pool size or if the pool size exceeds 16 donations then practicing virus inactivation methodology (72). The aforementioned methodology includes either utilizing pathogen-inactivated platelet as a source for HPL (under evaluation presently) or by executing a virus reduction step specifically while producing HPL.
Currently, there are no registered clinical trials with HPL for COVID-19. This further makes it imperative to consider it as a potent modality and clinical trials should be undertaken to widen its scope for the same. This would serve as a potential region of interest among researchers and regenerative medicine specialists across the globe to combat the pandemic caused by SARS-CoV-2.
Conclusions
The fundamentals and dynamics of platelets have been mentioned extensively in the medical literature. These lend potential substantiation to explore and extrapolate therapeutically. Considering this aspect; one such biologic is HPL. Research at present provides us with an insight into platelet lysate concerning its function and myriads of growth factors which can be directed in a supraphysiological dose for reparative and regenerative roles. Its role is extremely well adduced for acting as a supplement to the culture medium wherein replacing FBS but warrants a clearer picture in case of an immunological aspect. Emerging evidence regarding its repair and regenerative role preludes it as a potential plausible candidate to be extrapolated for COVID-19. Platelet lysate may emerge as a pivotal player provided investigations pace up in this context. The current focus should be on tight regulation controls and conduction of randomized controlled trials to establish the same.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/sci-2020-042). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization Model List of Essential Medicines. Geneva, Switzerland, WHO, 2019. Available online: https://www.who.int/medicines/publications/essentialmedicines/en/
- Ramesh R, Jeyaraman M, Prajwal GS. Autologous platelet-rich plasma therapy in orthopaedics: An update. Natl J Clini Orthop 2018;2:33-6.
- Henschler R, Gabriel C, Schallmoser K, et al. Human platelet lysate current standards and future developments. Transfusion 2019;59:1407-13. [Crossref] [PubMed]
- Burnouf T, Strunk D, Koh MB, et al. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016;76:371-87. [Crossref] [PubMed]
- Anitua E, Cugat R, Sanchez M. Platelet-rich plasma in orthopaedics and sports medicine. Chapter 1. First Edition. Switzerland, Springer, 2018:13-28.
- Geraldo RB, Sathler PC, Lourenço AL, et al. Platelets: still a therapeutical target for hemostatic disorders. Int J Mol Sci 2014;15:17901-19. [Crossref] [PubMed]
- Kisucka J, Butterfield CE, Duda DG, et al. Platelets, and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc Natl Acad Sci USA 2006;103:855-60. [Crossref] [PubMed]
- Varshney S, Dwivedi A, Pandey V. Antimicrobial effects of various platelet-rich concentrates-vibes from in-vitro studies-a systematic review. J Oral Biol Craniofac Res 2019;9:299-305. [Crossref] [PubMed]
- Mohamed HE, Asker ME, Kotb NS, El Habab AM. Human platelet lysate efficiency, stability, and optimal heparin concentration required in culture of mammalian cells. Blood Res 2020;55:35-43. [Crossref] [PubMed]
- Schallmoser K, Strunk D. Preparation of pooled human platelet lysate (pHPL) as an efficient supplement for animal serum-free human stem cell cultures. J Vis Exp 2009;1523. [Crossref] [PubMed]
- Seiffert D, Schleef RR. Two functionally distinct pools of vitronectin (Vn) in the blood circulation: identification of a heparin binding competent population of Vn within platelet alpha-granules. Blood 1996;88:552-60. [Crossref] [PubMed]
- Ahmed TA, Griffith M, Hincke M. Characterization and inhibition of fibrin hydrogel-degrading enzymes during development of tissue engineering scaffolds. Tissue Eng 2007;13:1469-77. [Crossref] [PubMed]
- Kraemer BF, Campbell RA, Schwertz H, et al. Novel anti-bacterial activities of beta-defensin 1 in human platelets: suppression of pathogen growth and signaling of neutrophil extracellular trap formation. PLoS Pathog 2011;7:e1002355 [Crossref] [PubMed]
- Cox D, Kerrigan SW, Watson SP. Platelets and the innate immune system: mechanisms of bacterial-induced platelet activation. J Thromb Haemost 2011;9:1097-107. [Crossref] [PubMed]
- Loppnow H, Bil R, Hirt S, et al. Platelet-derived interleukin-1 induces cytokine production, but not proliferation of human vascular smooth muscle cells. Blood 1998;91:134-41. [Crossref] [PubMed]
- Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nature Med 2007;13:463-9. [Crossref] [PubMed]
- Kopp HG, Placke T, Salih HR. Platelet-derived transforming growth factor-β down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res 2009;69:7775-83. [Crossref] [PubMed]
- Crawford SE, Stellmach V, Murphy-Ullrich JE, et al. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell 1998;93:1159-70. [Crossref] [PubMed]
- Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol 2000;18:767-811. [Crossref] [PubMed]
- Caux C, Massacrier C, Vanbervliet B, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med 1994;180:1263-72. [Crossref] [PubMed]
- Yang Y, Su Q, Grewal IS, et al. Transient subversion of CD40 ligand function diminishes immune responses to adenovirus vectors in mouse liver and lung tissues. J Virol 1996;70:6370-7. [Crossref] [PubMed]
- van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol 2000;67:2-17. [Crossref] [PubMed]
- Renshaw BR, Fanslow WC 3rd, Armitage RJ, et al. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med 1994;180:1889-900. [Crossref] [PubMed]
- Han S, Hathcock K, Zheng B, et al. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J Immunol 1995;155:556-67. [PubMed]
- Miller C, Stedra J, Kelsoe G, et al. Facultative role of germinal centers and T cells in the somatic diversification of IgVH genes. J Exp Med 1995;181:1319-31. [Crossref] [PubMed]
- Austrup F, Vestweber D, Borges E, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature 1997;385:81-3. [Crossref] [PubMed]
- Elzey BD, Tian J, Jensen RJ, et al. Platelet-mediated modulation of adaptive immunity: a communication link between innate and adaptive immune compartments. Immunity 2003;19:9-19. [Crossref] [PubMed]
- Elzey BD, Grant JF, Sinn HW, et al. Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J Leukoc Biol 2005;78:80-4. [Crossref] [PubMed]
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 2010;140:845-58. [Crossref] [PubMed]
- Langer HF, Weber C, Gawaz M. The platelet - thrombosis and beyond. Thromb Haemost 2013;110:857-8. [Crossref] [PubMed]
- Flaujac C, Boukour S, Cramer-Borde E. Platelets and viruses: an ambivalent relationship. Cell Mol Life Sci 2010;67:545-56. [Crossref] [PubMed]
- Assinger A. Platelets and infection - an emerging role of platelets in viral infection. Front Immunol 2014;5:649. [Crossref] [PubMed]
- Yeaman MR. Platelets: at the nexus of antimicrobial defence. Nat Rev Microbiol 2014;12:426-37. [Crossref] [PubMed]
- Solomon Tsegaye T, Gnirß K, Rahe-Meyer N, et al. Platelet activation suppresses HIV-1 infection of T cells. Retrovirology 2013;10:48. [Crossref] [PubMed]
- Tyner JW, Uchida O, Kajiwara N, et al. CCL5- CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat Med 2005;11:1180-7. [Crossref] [PubMed]
- Tang YQ, Yeaman MR, Selsted ME. Antimicrobial peptides from human platelets. Infect Immun 2002;70:6524-33. [Crossref] [PubMed]
- Polgar J, Matuskova J, Wagner DD. The P-selectin, tissue factor, coagulation triad. J Thromb Haemost 2005;3:1590-6. [Crossref] [PubMed]
- Elzey BD, Sprague DL, Ratliff TL. The emerging role of platelets in adaptive immunity. Cell Immunol 2005;238:1-9. [Crossref] [PubMed]
- Li N. Platelet-lymphocyte cross-talk. J Leukoc Biol 2008;83:1069-78. [Crossref] [PubMed]
- Gerdes N, Zhu L, Ersoy M, et al. Platelets regulate CD4(+) T-cell differentiation via multiple chemokines in humans. Thromb Haemost 2011;106:353-62. [Crossref] [PubMed]
- Shi G, Field DJ, Ko KA, et al. Platelet factor 4 limits Th17 differentiation and cardiac allograft rejection. J Clin Invest 2014;124:543-52. [Crossref] [PubMed]
- Sandri G, Bonferoni MC, Rossi S, et al. Platelet lysate embedded scaffolds for skin regeneration. Expert Opin Drug Deliv 2015;12:525-45. [Crossref] [PubMed]
- Del Fante C, Perotti C, Bonferoni MC, et al. Platelet lysate mucohadesive formulation to treat oral mucositis in graft versus host disease patients: a new therapeutic approach. AAPS PharmSciTech 2011;12:893-9. [Crossref] [PubMed]
- Pezzotta S, Del Fante C, Scudeller L, et al. Autologous platelet lysate for treatment of refractory ocular GVHD. Bone Marrow Transplant 2012;47:1558-63. [Crossref] [PubMed]
- Dellera E, Bonferoni MC, Sandri G, et al. Development of chitosan oleate ionic micelles loaded with silver sulfadiazine to be associated with platelet lysate for application in wound healing. Eur J Pharm Biopharm 2014;88:643-50. [Crossref] [PubMed]
- Ranzato E, Mazzucco L, Patrone M, et al. Platelet lysate promotes in vitro wound scratch closure of human dermal fibroblasts: different roles of cell calcium, P38, ERK and PI3K/AKT. J Cell Mol Med 2009;13:2030-8. [Crossref] [PubMed]
- Schallmoser K, Bartmann C, Rohde E, et al. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion 2007;47:1436-46. [Crossref] [PubMed]
- Strunk D, Lozano M, Marks DC, et al. International Forum on GMP-grade human platelet lysate for cell propagation: summary. Vox Sang 2018;113:80-7. [Crossref] [PubMed]
- Nguyen VT, Cancedda R, Descalzi F. Platelet lysate activates quiescent cell proliferation and reprogramming in human articular cartilage: Involvement of hypoxia inducible factor 1. J Tissue Eng Regen Med 2018;12:e1691-703. [Crossref] [PubMed]
- Wang TJ, Chen MS, Chou ML, et al. Comparison of three human platelet lysates used as supplements for in vitro expansion of corneal endothelium cells. Transfus Apher Sci 2017;56:769-73. [Crossref] [PubMed]
- Del Bue M, Riccò S, Conti V, et al. Platelet lysate promotes in vitro proliferation of equine mesenchymal stem cells and tenocytes. Vet Res Commun 2007;31:289-92. [Crossref] [PubMed]
- Klatte-Schulz F, Schmidt T, Uckert M, et al. Comparative Analysis of Different Platelet Lysates and Platelet Rich Preparations to Stimulate Tendon Cell Biology: An In Vitro Study. Int J Mol Sci 2018;19:212. [Crossref] [PubMed]
- Qi F, Qian S, Zhang S, et al. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun 2020;526:135-40. [Crossref] [PubMed]
- Li X, Geng M, Peng Y, et al. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 2020;10:102-8. [Crossref] [PubMed]
- Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-2. [Crossref] [PubMed]
- Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: From mechanisms to potential therapeutic tools. Virol Sin 2020;35:266-71. [Crossref] [PubMed]
- Lee YL, Lee LW, Su CY, et al. Virally inactivated human platelet concentrate lysate induces regulatory T cells and immunosuppressive effect in a murine asthma model. Transfusion 2013;53:1918-28. [Crossref] [PubMed]
- Golebiewska EM, Poole AW. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev 2015;29:153-162. [Crossref] [PubMed]
- Ghoshal K, Bhattacharyya M. Overview of platelet physiology: Its hemostatic and nonhemostatic role in disease pathogenesis. ScientificWorldJournal 2014;2014:781857 [Crossref] [PubMed]
- Fortunato TM, Beltrami C, Emanueli C, et al. Platelet lysate gel and endothelial progenitors stimulate microvascular network formation in vitro: tissue engineering implications. Sci Rep 2016;6:25326. [Crossref] [PubMed]
- Filardo G, Kon E, Roffi A, et al. Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc 2015;23:2459-74. [Crossref] [PubMed]
- Salamanna F, Veronesi F, Maglio M, et al. New and emerging strategies in platelet-rich plasma application in musculoskeletal regenerative procedures: General overview on still open questions and outlook. Biomed Res Int 2015;2015:846045 [Crossref] [PubMed]
- Yun SH, Sim EH, Goh RY, et al. Platelet Activation: The Mechanisms and Potential Biomarkers. Biomed Res Int 2016;2016:9060143 [Crossref] [PubMed]
- Aloui C, Prigent A, Sut C, et al. The signaling role of CD40 ligand in platelet biology and in platelet component transfusion. Int J Mol Sci 2014;15:22342-64. [Crossref] [PubMed]
- Burnouf T, Barro L, Nebie O, et al. Viral safety of human platelet lysate for cell therapy and regenerative medicine: Moving forward, yes, but without forgetting the past. Transfus Apher Sci 2019;58:102674 [Crossref] [PubMed]
- Karnieli O, Friedner OM, Allickson JG, et al. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy 2017;19:155-69. [Crossref] [PubMed]
- Astori G, Amati E, Bambi F, et al. Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: Present and future. Stem Cell Res Ther 2016;7:93. [Crossref] [PubMed]
- Pierce J, Benedetti E, Preslar A, et al. Comparative analyses of industrial-scale human platelet lysate preparations. Transfusion 2017;57:2858-69. [Crossref] [PubMed]
- Thieme D, Reuland L, Lindl T, et al. Optimized human platelet lysate as novel basis for a serum-, xeno-, and additive-free corneal endothelial cell and tissue culture. J Tissue Eng Regen Med 2018;12:557-64. [Crossref] [PubMed]
- Doucet C, Ernou I, Zhang Y, et al. Platelet lysates promote mesenchymal stem cell expansion: a safety substitute for animal serum in cell-based therapy applications. J Cell Physiol 2005;205:228-36. [Crossref] [PubMed]
- Altaie A, Owston H, Jones E. Use of platelet lysate for bone regeneration - are we ready for clinical translation? World J Stem Cells 2016;8:47-55. [Crossref] [PubMed]
- Henschler R, Gabriel C, Schallmoser K, Burnouf T, Koh MBC. Human platelet lysate current standards and future developments. Transfusion 2019;59:1407-13. [Crossref] [PubMed]
Cite this article as: Jeyaraman M, Muthu S, Khanna M, Jain R, Anudeep TC, Muthukanagaraj P, Siddesh SE, Gulati A, Satish AS, Jeyaraman N, Khanna V. Platelet lysate for COVID-19 pneumonia—a newer adjunctive therapeutic avenue. Stem Cell Investig 2021;8:11.