Quantitative analyses of myelofibrosis by determining hydroxyproline
Background
Myelofibrosis is the most serious form of myeloproliferative neoplasms (MPNs) (1-3). It results from the formation of scar-like tissues in the bone marrow which disrupts normal production of blood cells. The two other forms of MPNs are polycythemia vera (PV) and essential thrombocythemia (ET). PV is characterized by increased production of red blood cells, platelets, and white blood cells, whereas ET is manifest in the elevation of platelets only. Myelofibrosis can be presented as a primary condition which is named primary myelofibrosis (PMF). It can also be derived from PV and ET which are called post-PV or post-ET myelofibrosis. Patients with myelofibrosis have a very poor prognosis. So far, there is no effective treatment. JAK2V617F, a mutant form of tyrosine kinase JAK2, represents a major molecular defect in MPNs (4-8). It is found in over 95% of PV and over 55% of ET and PMF cases. Transgenic expression or knock-in of JAK2V617F in mice causes ET, PV, and myelofibrosis (9-15). Myelofibrosis is caused by clonal expansion of malignant hematopoietic cells which have profound impact on their stromal environment. Therefore, myelofibrosis is considered as a disease of both hematopoietic stem cells and stem cell niches (16).
Myelofibrosis is generally detected by staining of tissue sections with silver or trichrome (17). Since collagen types I and III are major components of fibrosis, immunohistochemistry for these two proteins is also commonly used. These staining methods give qualitative data, and subsequent scoring is rather subjective.
Hydroxyproline (4-hydroxyproline) is a non-proteinogenic amino acid formed by posttranslational hydroxylation of proline (18). It is a major component of collagen and has an important role in stabilizing the helical structure. Because hydroxyproline is largely restricted to collagen, the level of hydroxyproline can be considered as an indicator of collagen content. In fact, the hydroxyproline content was found to be correlated with bone marrow fibrosis, and hydroxyproline in urine and serum has been used as a marker to monitor conditions that increase collagen turnover and bone resorption (19-22). In this study, we developed a quantitative assay of bone marrow myelofibrosis in JAK2V617F transgenic mice by determining hydroxyproline.
Materials and methods
Materials
Richard-Allan Scientific Decalcifying Solution which contains EDTA and dilutes hydrochloric acid was purchased from Thermo Scientific. Reticulum Stain Kit and all other chemical reagents were from Sigma-Aldrich.
Mice
JAK2V617F transgenic mice were generated by expressing human JAK2V617F in the hematopoietic system under the control of the vav-1 promoter (9). These mice have been crossed with wild type C57BL/6 mice for over 10 generations. Homozygous line A JAK2V617F mice were used in this study as previously described (23-25). Wild type C57BL/6 mice were used as control. Animals were housed in ventilated cages under standard conditions. This study was carried out under an approved protocol in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Mouse tissue collection and histochemical staining
Mice were put under deep anesthesia through inhalation of isoflurane and terminated by terminal blood collection and open heart surgery. Femurs were dissected and fixed with 10% neutral buffered formalin overnight at room temperature. Following decalcification over night at room temperature by using the Richard-Allan Scientific Decalcifying Solution, the tissue was embedded in paraffin, and 5 µm tissue sections were cut for histochemical staining. Reticulin staining was carried out by using a kit from Sigma-Aldrich following a standard protocol (9,24). Slides were viewed with an Olympus BX-51 upright microscope equipped with U Plan Fluorite objectives. Images were acquired using a DP71 digital camera and processed with the Adobe Photoshop software. Quantification of histochemical staining images was done by using the NIH ImageJ program.
Acid hydrolysis and hydroxyproline assay
Following formalin fixation and EDTA decalcification, bone marrow tissues were dissected from the bone under a stereo microscope. Bone marrow tissues (usually 5-8 mg, wet weight, from a whole femur) were collected and hydrolyzed with 0.3 mL of 6 M HCl in O’-ring screw-capped tubes at 120 °C on a heating block for 3 h. Total protein in the bone marrow was determined by measuring OD280nm and OD260nm of the hydrolysates and calculated based on the Warburg and Christian formula (protein concentration at mg/mL =1.55×OD280nm -0.76×OD260nm). Hydroxyproline assays were performed according to an established protocol (26,27). In brief, the bone marrow hydrolysates were diluted 10-fold in water, and 1-10 µL diluted samples were taken and dried in a speed vacuum concentrator. After the oxidation with chloramine T at room temperature for 5 min, Ehrlich’s reagent (p-dimethylaminobenzaldehyde dissolved in n-propanol/perchloric acid) was added and further incubated for 90 min at 60 °C. The absorbance of reddish purple complex was measured at 560 nm using a spectrophotometer. Standard hydroxyproline was analyzed in the same way, and hydroxyproline contents in the samples were calculated based on standard curves and normalized against total protein concentrations.
Statistical analysis
Statistical analyses were performed using the GraphPad Prism program. Differences of samples between two groups were accessed using t tests. P values less than 0.05 (2-tailed) were considered significantly different.
Results
Development of an assay for detection of myelofibrosis by analyzing hydroxyproline in the bone marrow
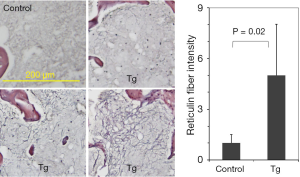
As reported in our earlier studies, JAK2V617F transgenic mice developed bone marrow fibrosis (9). Figure 1 shows typical reticulin staining results of bone marrow sections obtained from 40-week-old control and transgenic mice. While reticulin fibers were clearly seen in JAK2V617F transgenic mice, they were essentially absent in the control mice. However, the fibers were not uniformly distributed in the bone marrow of transgenic mice. The intensity of reticulin staining varied substantially in different sections and areas. Although analyses of multiple images by using the ImageJ computer software provided semi-quantitative data, the error is usually large. Therefore, reticulin staining is mainly a qualitative assay and may not be suitable for assessing incremental progression of myelofibrosis in transgenic mice.
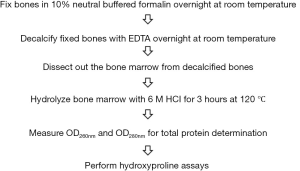
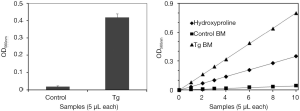
Since collagen is the major component of reticulin fibers and hydroxyproline is a largely restricted to collagen, we thought to develop a method to detect bone marrow myelofibrosis by analyzing hydroxyproline contents. A flowchart of the method is shown in Figure 2. The procedure contains six simple steps. The two initial steps can be conducted in conjunction with histochemical analyses and were found to be essential for collection of clean and representative bone marrow tissues. The remaining steps follow the standard protocol for chemical analysis of hydroxyproline in proteins. The hydroxyproline analysis results obtained with bone marrow from same mice described in Figure 1 are shown in Figure 3. The results revealed sharp contrast between control and transgenic mice, and the data was representative of a whole femur with a very negligible margin of error. The assay is apparently highly sensitive because a bone marrow sample corresponding to 1/600 of total materials in a mouse femur is sufficient to produce a readout shown in Figure 3A. We further demonstrated the linearity of the assay by using different volumes of bone marrow hydrolysates and standard hydroxyproline (Figure 3B). The data suggest that the method is able to cover a large range for hydroxyproline assays in the bone marrow.
Revelation of age-dependent development of myelofibrosis in JAK2V617F transgenic mice
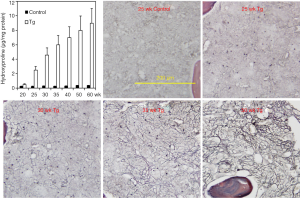
We then employed the hydroxyproline detection method to analyze bone marrow myelofibrosis in JAK2V617F mice of different ages ranging from 20 to 60 weeks. Hydroxyproline levels were normalized against total protein contents determined by measuring OD280nm and OD260nm of bone marrow hydrolysates. The data shown in Figure 4 demonstrated a gradual increase in myelofibrosis in the transgenic mice as they aged. In contrast, control mice displayed a minimal level of hydroxyproline regardless of their ages. Overall, the hydroxyproline analysis data correlated very well with the reticulin staining. However, it should be indicated that at 20 weeks of age, hardly any myelofibrosis was seen with reticulin staining (not shown) but hydroxyproline assay detected a significant difference between control and transgenic mice. Together, the data provide quantitative data demonstrating age-dependent development of myelofibrosis in JAK2V167F transgenic mice. We thus provide a useful tool to detect myelofibrosis at earlier stages and to follow the progression of the disease quantitatively.
Discussion
In the present study, we have developed a new method for detecting bone marrow myelofibrosis by analyzing hydroxyproline contents. The method is highly sensitive and accurate. It provides more accurate, representative, and quantitative information than histochemical analyses. We believe that this method should find wide applications for analyzing the progression of myelofibrosis and efficacy of drug treatment.
For analysis of bone marrow tissues, fixation and decalcification are necessary to collect clean and representative samples. This is because bone marrow cells can’t be recovered effectively by simple flushing or cracking especially for those with extensive myelofibrosis. After fixation and decalcification, fixed bone marrow tissues can be easily recovered in a whole piece. This works for small as well as big bones. We have also tried whole bones for direct acid hydrolysis followed by hydroxyproline determination. However, the bone tissue itself and associated cartilage gave rise to a very high background, making the detection inconsistent. Nonetheless, the method can be used to analyze myelofibrosis in the spleen with fresh, frozen, or formalin-fixed tissues. Despite a significant background caused by abundant vascular structures, JAK2V617F-induced spleen myelofibrosis is clearly reflected by an increase in hydroxyproline contents (data not shown). It should be pointed out that formalin fixation of tissues does not affect detection of hydroxyproline.
Acknowledgements
Funding: This work was supported by grants from Oklahoma Center for the Advancement of Science & Technology and the MPN foundation and a pilot grant from the Stephenson Cancer Center of University of Oklahoma.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Levine RL, Gilliland DG. Myeloproliferative disorders. Blood 2008;112:2190-8. [PubMed]
- Tefferi A. The history of myeloproliferative disorders: before and after Dameshek. Leukemia 2008;22:3-13. [PubMed]
- Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med 2006;355:2452-66. [PubMed]
- Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005;365:1054-61. [PubMed]
- Levine RL, Wadleigh M, Cools J, et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005;7:387-97. [PubMed]
- James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144-8. [PubMed]
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005;352:1779-90. [PubMed]
- Zhao R, Xing S, Li Z, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem 2005;280:22788-92. [PubMed]
- Xing S, Wanting TH, Zhao W, et al. Transgenic expression of JAK2V617F causes myeloproliferative disorders in mice. Blood 2008;111:5109-17. [PubMed]
- Shide K, Shimoda HK, Kumano T, et al. Development of ET, primary myelofibrosis and PV in mice expressing JAK2 V617F. Leukemia 2008;22:87-95. [PubMed]
- Tiedt R, Hao-Shen H, Sobas MA, et al. Ratio of mutant JAK2-V617F to wild-type Jak2 determines the MPD phenotypes in transgenic mice. Blood 2008;111:3931-40. [PubMed]
- Mullally A, Lane SW, Ball B, et al. Physiological Jak2V617F expression causes a lethal myeloproliferative neoplasm with differential effects on hematopoietic stem and progenitor cells. Cancer Cell 2010;17:584-96. [PubMed]
- Marty C, Lacout C, Martin A, et al. Myeloproliferative neoplasm induced by constitutive expression of JAK2V617F in knock-in mice. Blood 2010;116:783-7. [PubMed]
- Akada H, Yan D, Zou H, et al. Conditional expression of heterozygous or homozygous Jak2V617F from its endogenous promoter induces a polycythemia vera-like disease. Blood 2010;115:3589-97. [PubMed]
- Li J, Spensberger D, Ahn JS, et al. JAK2 V617F impairs hematopoietic stem cell function in a conditional knock-in mouse model of JAK2 V617F-positive essential thrombocythemia. Blood 2010;116:1528-38. [PubMed]
- Lataillade JJ, Pierre-Louis O, Hasselbalch HC, et al. Does primary myelofibrosis involve a defective stem cell niche? From concept to evidence. Blood 2008;112:3026-35. [PubMed]
- Kuter DJ, Bain B, Mufti G, et al. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol 2007;139:351-62. [PubMed]
- Gordon MK, Hahn RA. Collagens. Cell Tissue Res 2010;339:247-57. [PubMed]
- Charron D, Robert L, Couty MC, et al. Biochemical and histological analysis of bone marrow collagen in myelofibrosis. Br J Haematol 1979;41:151-61. [PubMed]
- Wang JC, Aung MK, Tobin MS. Urinary hydroxyproline excretion in myelofibrosis. Blood 1980;55:383-5. [PubMed]
- Pagani F, Francucci CM, Moro L. Markers of bone turnover: biochemical and clinical perspectives. J Endocrinol Invest 2005;28:8-13. [PubMed]
- Need AG. Bone resorption markers in vitamin D insufficiency. Clin Chim Acta 2006;368:48-52. [PubMed]
- Jin X, Zhao W, Shi K, et al. Generation of a new congenic mouse strain with enhanced chymase expression in mast cells. PLoS One 2013;8:e84340. [PubMed]
- Shi K, Zhao W, Chen Y, et al. Cardiac hypertrophy associated with myeloproliferative neoplasms in JAK2V617F transgenic mice. J Hematol Oncol 2014;7:25. [PubMed]
- Jin X, Zhao W, Kirabo A, et al. Elevated levels of mast cells are involved in pruritus associated with polycythemia vera in JAK2V617F transgenic mice. J Immunol 2014;193:477-84. [PubMed]
- Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta 1967;18:267-73. [PubMed]
- Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem 1996;29:225-9. [PubMed]
Cite this article as: Zhao W, Ho WT, Zhao ZJ. Quantitative analyses of myelofibrosis by determining hydroxyproline. Stem Cell Investig 2015;2:2.