TNF-α, a good or bad factor in hematological diseases?
Introduction
Hematological malignancies are a group of diseases involved with blood, bone marrow (BM) and lymph nodes, including acute myeloid leukemia (AML), lymphoma, multiple myeloma (MM), and so on. Accumulating evidences have shown that inflammatory environment and cytokines play a pathological role in the onset and progress of hematological malignancies. Tumor necrosis factor-alpha (TNF-α), one of the best characterized cytokines, was discovered in the mouse serum during endotoxemia and recognized for its anti-tumor activity (1). In the 1980s, TNF-α was also characterized as cachectin (2) for its role in the wasting syndrome, and as T-lymphocyte differentiation factor (3). Cloning of TNF gene in 1984 (4) led to the era of clinical experimentation. TNF gene is located on chromosome 6p21.3 and is mainly expressed by activated macrophages, NK-cells and T-lymphocytes, even other cell types (e.g., fibroblasts, astrocytes, Kupffer cells, smooth-muscle cells, keratinocytes, and tumor cells) have been shown to express it (5).
Regulation of TNF-α biosynthesis has been observed mainly at the level of transcription, mRNA export, post-transcription and translation. Induction of TNF-α gene transcription is cell-type specific and stimulus specific, which involves the recruitment of distinct sets of transcription factors to the promoter region, including NF-κB, Sp1, NF-AT, ATF-2, c-jun and CREB, among others (6). Post-transcriptional regulation may influence the level of mRNA translation and is mediated in part by specific and conserved sequences within the mRNA. Such sequences include the adenosyl-uridyl-rich elements (AREs) and micro-RNA (miRNA) target sites present in the 3’-untranslated region. ARE bind to specific proteins, which regulate mRNA stability or translation in response to both external and internal stimuli. miRNAs are small noncoding RNA molecules that recruit a protein complex to a complementary target mRNA, which results in translation repression or degradation of mRNA (7).
TNF-α is a highly pleiotropic cytokine involved in spectrum of physiological processes that control inflammation, anti-tumor responses and homeostasis through engagement of two cognate single-pass transmembrane glycoprotein receptors: TNF-R1 and TNF-R2. This cytokine can be either membrane-bound or secreted by cells. TNF-α is first synthesized as a 26-kDa (233 amino acids) precursor membrane-bound TNF-α (tmTNF). And this pro-TNF-α is proteolytically processed by the TNF-converting enzyme (TACE) to release a soluble 17-kDa TNF-α (sTNF) homotrimer. TACE, a member of a-disintegrin-and-metalloproteinase (ADAM) family, can also release TNF receptors from the cell surface: these circulating cytokine-binding proteins represent an important mechanism of regulation for the biological activity of soluble TNF (8). Specifically, the soluble forms of both receptors, s-TNF-R1 and -R2, bind TNF-α with high affinity, which transduces or inhibits TNF-α biological activity. At high concentrations, they inhibit TNF bioactivity by competing with the membrane receptors. At low concentrations, they could stabilize the trimeric TNF-α structure increasing its half-life and acting as reservoir of TNF-α (9).
Soluble TNF-α is best known for its role in leading immune defenses to protect a localized area from invasion or injury but it is also involved in controlling whether target cells live or die. In general, TNF largely relies on TNFR1 for apoptosis and on TNFR2 for any function related to T-cell survival. Various defects in the TNF signaling pathway that act through TNFR2 and NF-κB, in autoreactive T cells, have been found in both human and in mouse models of autoimmune disorders, including Crohn’s disease, Sjogren’s syndrome, multiple sclerosis, systemic lupus erythematosus, ankylosing spondylitis and type 1 diabetes (10-13). The role of TNF-α in cancer therapy is debated. At one hand, a large body of evidence supports TNF’s antineoplastic activity, such as direct cytotoxic effects (14), dose-dependent reduction of tumor blood flow (15) and enhancing antitumor cytolytic activity and production of cytotoxicity-related proteins (e.g., IFN-γ, TIA-1) by NK cells (16,17). On the other hand, some pre-clinical findings suggest that TNF may promote cancer development and progression. TNF may act as an autocrine tumor growth factor by promoting cell survival through the activation of the NF-κB-, PI3K-PKB/AtK- and MAPK-dependent anti-apoptotic pathways. The cytokine upregulates the expression of positive cell-cycle regulators (Ras, C-Myc) and decreases the level of Cdk-inhibitors (18). What’s more, some investigators even showed that TNF is crucial for promoting metastasis (19,20).
Transmembrane TNF-α exerts its biological function in a cell-to-cell contact fashion, which is distinct from the feature of soluble TNF-α. Transmembrane TNF-α not only acts as a ligand by binding to TNF-α receptors, but also functions as a receptor that transmits outside-to-inside (reverse) signals back into the transmembrane TNF-α-bearing cells (TNF-α-producing cells) (21). It is therefore considered that transmembrane TNF-α plays a critical role in local inflammation (22). Specifically, as a ligand, transmembrane TNF-α expressed on various cell types would contribute to the physiological as well as pathological response in health and diseases. As a receptor, transmembrane TNF-α contributes to the pleiotropy of the cytokine and its fine-tuning of immune response (21).
TNF-α signaling transduction
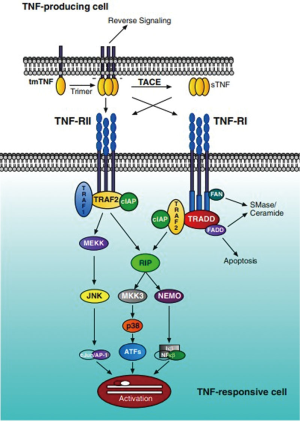
TNF-α transduces its signal through two distinct receptors, referred to as TNF-R1 (also called TNFRp55/p60, 60 kDa) and TNF-R2 (also called TNFRp75/p80, 80 kDa) (23,24). The two cognate TNF-α receptors are differentially expressed and controlled. TNF-R1 is constitutively expressed on most cell types, with the exception of erythrocytes. Conversely, TNF-R2 expression is induced upon inflammatory stimulation and is limited to hematopoietic lineage cells. Due to the relatively restricted expression of TNF-R2, it has been generally considered that the majority of the biological effects mediated by TNF-α are achieved through its interaction with TNF-R1 (25). Initially, TNF-R1 binds the TNF homotrimer to the extracellular domain of the receptor, which induces TNF-R1 trimerization and the release of the inhibitory protein silencer of death domains (SODD) from TNF-R1 intracellular death domain (DD). After SODD release, TNF-R associated DD (TRADD) can bind to the DD of TNF-R1 and then recruit additional adaptor proteins, such as receptor-interacting protein (RIP), TNF-R associated factor (TRAF)-2 and Fas-associated DD (FADD). When caspase-8 is recruited by FADD to the death inducing signaling complex (DISC), it becomes activated, presumably by self-cleavage, and initiates a protease cascade leading to death. While TRAF-2 recruits inhibitor-of-apoptosis protein-1 (IAP-1) and IAP-2, it starts a pathway resulting in the phosphorylation/activation of transcription factors (c-Jun, c-Fos, ATF-2) via the activation of MAPK (JNK, p38MAPK and ERK). TRAF-2 also activates the protein kinase RIP, which is critical to the activation of NF-κB (Figure 1) (26). Different from TNF-R1, TNF-R2 can also mediate these above pathways by directly binding with TRAF2 along with TRAF1 to elicit intracellular crosstalk between the receptors (27,28). What’s more, even TNF-R2 lacks a DD, its effect on programmed-cell-death (PCD) via NF-κB and JNK pathway activation (5) or TRAF-2 inhibition (29) cannot be ruled out. Considering another function of TNF-R2, accumulating evidence have shown that signaling through the TNF-R2 influences a number of pro-inflammatory responses, including the activation of T cells (30), myofibroblasts (31), inhibition of angiogenesis and tumor suppression (32).
TNF-α in regulation of hematopoiesis
A complex interplay of various cytokines has been implied in maintaining normal hematopoiesis. Growth factors such as erythropoietin (EPO), granulocyte macrophage colony stimulating factor (GM-CSF), granulocyte colony stimulating factor (G-CSF) and interleukin-3 (IL-3) promotes the differentiation of erythroid and myeloid progenitors (33). On the other hand, interferons, interleukins and TGF-beta have inhibitory actions on hematopoietic stem cells. It is conceivable that an imbalance between the action of inhibitory and stimulatory cytokines can lead to increased myelo-suppression and BM failure (34). TNF-α has been shown to be a bifunctional regulator of the growth of hematopoietic stem and progenitor cells, depending on the growth factors used as supplements (35). Researchers have reported that mouse TNF-α inhibits the growth of high proliferative potential colony-forming cells (HPPCs) from primitive Lin–Sca-1+ hematopoietic progenitor cells (HPCs) regardless of the growth factors used for the experiments (36). Additionally, in vitro experiments have demonstrated detrimental effects of TNF-α on hematopoietic cell function (23). However, some studies have shown that TNF-α can stimulate the secretion of IL-3 and GM-CSF in supplemented cultures of human CD34+ HPCs during short-term culture (37). Another study reported that TNF-α can mediate up-regulation of GM-CSF receptors on DC precursors (38). Moreover, TNF-α has been demonstrated to stimulate IL-3 to mediate DC development from human cord blood (39). Mizrahi et al. demonstrated that TNF-α is a modulator of hematopoietic activity in fresh umbilical cord blood and cryopreserved mobilized peripheral blood samples, with significant tropic activity mediated by TNF-R1 and transduced through caspase-8 (40). Pearl-Yafe et al. showed that TNF-α plays a tropic role early after transplantation, which is essential to successful progenitor engraftment. Transplantation of lineage-negative BM cells from TNF receptor-defective mice into wild-type recipients showed defective early engraftment and loss of durable hematopoietic contribution upon recovery of host hematopoiesis (41). All of these results imply that TNF-α can act as inducers of hematopoiesis in the BM microenvironment.
TNF-α in hematological diseases in human and animal models
Acute myeloid leukemia (AML)
AML represents a group of clonal hematopoietic stem cell malignant diseases which are characterized by three different features. The first feature is that hematopoietic cells arrest at a certain level of differentiation. Secondly, progenitor cells continue to proliferate thereby displacing normal hematopoiesis. The third feature is that immature cells prematurely leave the BM and infiltrate peripheral tissues (42).
TNF-α is a major effector and regulatory cytokine with a pleiotropic role in the pathogenesis of several immune-regulated diseases and hematologic malignancies, including AML (43). TNF-α stimulates the proliferation of dividing cells causing hypercellularity, or induces apoptosis in their maturing progeny which results in pancytopenia (44). It can be produced not only by macrophages but also by activated NK cells, neutrophils, CD4+ T helper 1 (Th1) cells, CD8+ T cells and even AML blasts (45-47). TNF-α has conflicting roles in cancer. TNF-α can act directly or indirectly as an autocrine tumor growth factor, while many studies also showed that TNF-α induces apoptosis of tumor cells (48,49). About the specific function of TNF-α in AML pathogenesis, onset or progress, beneficial or negative, there are discrepancies observed in the different studies. Study has found that mean level of TNF-α was significantly higher in AML patients than in the healthy volunteers (50). And high serum TNF-α level is an adverse prognostic factor for survival and event-free survival in patients with AML (51). On the contrary, in other study, the analysis of AML patients at diagnosis or during relapsed disease showed that TNF-α was significantly elevated in chronic leukemia and acute lymphoblastic leukemia but not in AML (52). The discrepancies observed are probably due to the existence of high inter-individual variations. For the hematopoiesis, study showed that TNF-α may act indirectly by rendering leukemic cell cytotoxic toward residual normal hematopoietic cells, and that mechanism may at least contribute to hematopoiesis insufficiency (53). Ismair et al. presented the existence of an autocrine TNF-α-loop in the APL (AML-M3) cell line NB4, which selectively upregulated matrix metalloproteinase-9 (MMP-9) expression via the TNF-R1/NF-κB pathway, however has no influence on the expression of tissue inhibitor of metalloproteinased-1 (TIMP-1) (54). Considering TNF-α levels are significantly increased in the serum of AML patients (55) and that TNF-α is constitutively expressed in myeloid leukemia (56), the TNF-α-induced MMP-9 expression may be important in the pathophysiology of the premature egress of leukemic cells from the BM and their dissemination into peripheral tissues (54). Additionally, Reuter et al. showed that with TNF-α concentration rising, AML patients experienced increased fatigue, whereas improving fatigue over time was associated with reductions in TNF-α level (48). All these studies imply that TNF-α may play a negative role in the AML pathophysiology, disease metastasis and progress. Further researches may be needed to reveal the specific role of TNF-α and critical signaling pathway in AML. And due to the strong side effects of chemotherapeutics, TNF-α and the signaling pathway may be as a target to solve this problem.
Myelodysplastic syndromes (MDS)
MDS represent a heterogeneous group of clonal myelopoietic stem cell disorders characterized by persistent peripheral cytopenia with morphological and functional abnormalities of hematopoietic cells, often contrasted by BM hypercellularity, with an increased risk of transformation into AML (44). Based on the degree of cytopenia and malignant potential, MDS can be classified into low or high grade subtypes, using the international prognostic scoring system (57). In low grade MDS, marrow hypercellularity and peripheral cytopenia are commonly seen due to upregulated apoptosis in the progenitor stem cells. However, decreased apoptosis is seen during transformation to high risk MDS, which often manifests with an increase in myeloblasts (58).
It is interesting to note that in MDS patients serum TNF-α levels (59), as well as the expression of TNF-α in the BM, are elevated (60). And Sawanobori et al. observed increased TRADD/FADD expression and decreased expression of TRAF-2 (61). Indeed, a molecular study on frozen tissue (61) convincingly showed increased expression of TNF-R1, which is involved in pro-apoptotic signaling (62), on myeloid and erythroid progenitor cells and T-lymphocytes in refractory anemia at the RNA as well as protein level, but a predominance of TNF-R2 in MDS with higher proliferative activity and AML. Even more, the authors demonstrated potentially functioning pro-apoptotic TNF-associated down-stream pathways in refractory anemia, contrasted by lacking pro-apoptotic cascade members in AML (61). Studies also showed that the progression of MDS to acute leukemia has been correlated with upregulation of NF-κB, altered expression of adaptor molecules such as flice inhibitory protein (FLIP), and enhanced activity of anti-apoptotic members of the Bcl-2 and the inhibitors of apoptosis protein (IAP) families. However, considering that TNF-α levels are more commonly elevated in early stage disease, where NF-κB activity may be low, suggesting that TNF-α do not strictly correlated with NF-κB activity. Therefore, autocrine TNF-α stimulation is probably not the sole mechanism involved in the constitutive NF-κB activation (63).
Moreover, other studies have also demonstrated that TNF-α may play a role in the pathogenesis of MDS. Researcheres showed that polymorphism associated with increased expression of the cytokine TNF-α is overrepresented in the MDS population suggesting that increased TNF-α activity may contribute to the susceptibility and/or pathogenesis of MDS. Results indicated that high-expressing TNF-308 and TNF-238 genotypes were independently associated with neutropenia and severity of anemia, respectively, in a large cohort of treatment naïve, de novo MDS patients (64). It has been increasingly recognized that cancer initiation and progression is not solely a cancer cell autonomous process, but tumor progression microenvironment may significantly contribute to define tumor characteristics. Ishibashi et al. has provided a disease-progression model in which overproduction of TNF-α in the microenvironment is the primary event. TNF-α activates NF-κB that in turn induces B7-H1 molecule expression on MDS blasts, which generates a bifunctional signal inducing T-cell apoptosis and enhancing blast proliferation. And the latter may provide more opportunity for developing secondary genetic changes (65). Meanwhile, TNF-α-induced gene expression in human marrow stroma may provide clues to the pathophysiology of MDS. Stirewalt et al. suggested that TNF induced a complex set of proinflammatory and pro-apoptotic signals in stroma cells that promote apoptosis in malignant myeloid clones, which exhibited as increased mRNA expression of proinflammatory cytokines (e.g., IL-6, IL-8 and IL-32) and pro-apoptotic genes (e.g., BID) but decreased mRNA expression of anti-apoptotic genes (e.g., BCL2L1) (66).
Significant progress has been made in understanding the role of TNF-α in MDS. However, the clinical application of TNF-α antagonist showed gloomy results. For etanercept, a TNF-α inhibitor was first tested in a phase II study by Deeg et al. and interestingly there was no correlation observed between the pretreatment TNF-α levels and hematologic response (67). Similar to etanercept, the therapeutic efficacy of infliximab was evaluated. A low response rate was noted with both the doses which imply that TNF-α blockade alone might be an insufficient therapeutic strategy in MDS (68,69). And the drug combination therapy may be a good choice. In a phase II trial, Scott et al. showed that for patients with low grade MDS, a combination of anti-thymocyte globulin (ATG) and etanercept results in higher response rate than observed with ATG alone (69). The specific function of TNF-α, its action mechanism and the downstream pathways are needed to be elucidated, which can provide new insight to the treatment.
Fanconi anemia (FA)
FA is an autosomal recessive disorder characterized by multiple congenital abnormalities, BM failure, and susceptibility to cancer (70). Ninety percent of all deaths result from complications of a progressive BM failure or a generally late malignant myeloid transformation, especially AML (71). To date, 13 complementation groups (FANC A,B,C,D1,D2,E,F,G,I,J,L,M,N) have been identified and the cDNAs of 9 of these genes that collectively account for over 95% of all FA patients have been cloned (72-74). FA cells are known to have an increased propensity to undergo apoptosis in response to both DNA-damaging agents and inhibitory cytokines (75). Cytokine hypersensitivity of FA hematopoietic cells to apoptotic cues has been proposed as a major factor in the pathogenesis of BM failure in three FA mouse models (Fanca-/-, Fancc-/-, and Fancg-/-) (76,77).
Studies have shown that patients with FA have abnormally high levels of proinflammatory TNF-α, low levels of natural killer cell activity and reduced lymphocyte counts, and are highly susceptible to bacterial infection (78-80). However, no anomalies were detected at the gene and mRNA levels for TNF-α (80). Studies also demonstrated that many of the cellular characteristics of FA match with the detrimental effects ascribed to the overexpression of TNF-α. It has already been mentioned that DNA strand breakage activity (81,82) may contribute to the hypersensitivity to DNA damage of FA cells; the partial correlation of this FA feature by treatment with antibodies against the TNF-α supports this view. Moreover, the spontaneous chromosomal fragility, the G2 phase delay typical of FA cells (83), and the general poor growth if FA cell in culture (84) may be, at least partially, related to TNF-α overexpression. In view of overexpression deleterious in vivo activities observed not only in vitro (85) but also, as recently shown, in patients (86), TNF-α may play a role in FA BM failure and in the subsequent onset of pancytopenia.
Recently, Sejas et al. showed that TNF-α is responsible in part for LPS-induced hematopoietic suppression and subsequent septic shock. The in vivo finding presented here clearly demonstrated that deletion of TNF-α gene or neutralization of TNF-α in LPS-treated Fancc-/- mice effectively rescued progenitor growth and hematopoietic reconstitution (87). This finding underscored pathogenic roles of TNF-α in clinical manifestations in BM failure-related diseases including FA. The study also demonstrated that the production of intracellular reactive oxygen species (ROS) by TNF-α at inflammatory sites caused DNA damage (23), contributed to the progression of cancer-related BM failure diseases like FA, caused carcinogenic mutations which may promote clonal evolution in MDS and leukemic transformation (87). Additionally, studies showed that TNF-α exposure initially inhibited profoundly the expansion of Fancc-/- stem progeny. However, longer-term exposure of these cells promoted the outgrowth of TNF-α-resistant, cytogenetically abnormal clones, which can lead to AML after being transplanted into syngeneic WT mice (88).
All these results may imply that TNF-α plays a negative role in the pathogenesis of FA. For the clinical application, some report concluded that Etanercept is well tolerated and can be safely administrated to FA patients. However, there was no evidence of an effect of Etanercept on hematopoiesis (89). Further exploration of TNF-α functional pathway, correlation with other pathogenic factor and anti-inflammatory therapies in FA are needed.
Multiple myeloma (MM)
MM is an incurable B-cell neoplasm characterized by an uncontrolled expansion of neoplastic plasma cells, called myeloma cells, in the BM. Disease course is often divided into three stages. Firstly, a plasma cell clone acquires abnormally low apoptosis rate and accumulates, which were clinically recognized either as MM or monoclonal gammopathy of unknown significance. Secondly, clonal expansion occurs in close proximity to BM stroma cells that provide multiple growth (increased mitosis) and survival (decreased apoptosis) signals, among which prominently are TNF and IL-6. In the terminal phase the malignant clone acquires marrow independence and consequent metastasis to extra-medullary sites (90,91).
In early and middle stages of MM, accretion is more by abnormally low apoptosis rate than high mitotic rate (90-92). Lower apoptosis rate is mediated in part by TNF (91-93). TNF does so by several pathways, one of which is activation of NF-κB (94). In MM, there is evidence that NF-κB activity promotes growth, immortalization and survival of tumor cells as well as angiogenesis (95). TNF is a strong activator of the classical NF-κB pathway and is itself regulated by this pathway (96). Stimulating autocrine production of IL-6, TNF-α also induces myeloma cell cycle and promotes the long-term growth of malignant plasma cell lines (97,98). Study also observed reduction of TNF-α could lead to down-regulation of IL-6 production, which is the main growth factor for myeloma cells (98). Moreover, TNF-α participated in the migration of MM. Study showed that TNF-α-induced up-regulation of monocyte chemoattractant protein (MCP)-1 in myeloma cells correlated with migration, and blocking MCP-1 abrogated the enhanced migration induced by TNF-α stimulation (93).
Considering a certain degree of familial aggregation has been observed, genetic factors can be involved in the pathogenesis and the evolution of MM (99). Studies showed that the presence of TNF-α rs1800629_A allele, associated to increased level of TNF-α (100), could contribute to accentuate inflammatory status associated to ageing and age-related morbidity and mortality. And reports also showed that this SNP correlated with a shorter progression free survival (PFS) of patients over 60s (101).
In view of the negative role of TNF-α in MM pathogenesis, clinical application of chemotherapeutics that target the TNF-α and related cytokines was observed. Thalidomide (THAL) is a drug that was used in the early 1960s as a sedative, but it was quickly withdrawn because of its teratogenic effects. However recently, a growing number of autoimmune, inflammatory and cancer diseases have also been reported to respond to THAL treatment including MM (102-104). Dmoszynska et al. showed that some of antimyeloma effects of THAL were related to a decreased production of TNF-α and IL-6 (98). However, the application of Etanercept showed a poor outcome that shortened survival (105). Kast’s research results demonstrated that etanercept resulted in increased levels of TNF-α and possibly aggravated the condition. The explanation is that since TNF-R2 signaling tends to be more anti-apoptotic and activating of NF-κB, than is TNF-R1, and TNF-R1 tends to be more pro-apoptotic than is TNF-R2, by inactivating soluble TNF while leaving transmembrane TNF signaling relatively unchanged, Etanercept changed the balance in TNF signaling from TNF-R1 towards TNF-R2 weighting. Anti-apoptosis and TNF synthesis would have been up-regulated by that shift (105). Additionally, recently a report showed a case of a 53-year-old patient who had pyoderma gangrenosum (PG) and monoclonal gammopathy of uncertain significance (MGUS). After treatment with infliximab for the PG, he developed myeloma. The course of events in this case suggested that infliximab facilitated the progression from MGUS to myeloma (106). Further researches were needed to explore the specific role of TNF-α, whether physiological or pathological, and come up with an effective regime to solve the disease.
Lymphoma
Hodgkin lymphoma (HL) is a peculiar form of malignancy in which the clonal B-cell population, the so-called mononuclear Hodgkin and multinuclear Reed-Sternberg (HRS) cells as well as their variants, are responsible for less than 1% of tumor bulk (107). Most of the cells are reactive elements, such as lymphocytes, plasma cells, eosinophils, histiocytes, and fibroblasts. It seems probably that the major clinical and histopathological features of HD reflect the production of cytokines by either the neoplastic or the reactive cell populations.
TNF-α is a central mediator of inflammatory processes, generating a cytokine cascade (108). In addition, TNF-α has been recently identified to participate in the development and function of normal lymphoid tissues (109). Two receptors, TNF-R1 and TNF-R2 mediate the effects of TNF-α on target cells through several pathways leading to cell activation, proliferation or apoptotic death. Warzocha et al. demonstrated plasma levels of TNF, TNF-R1 and TNF-R2 were higher in HD patients than in healthy controls. Plasma levels of TNF, TNF-R1 and TNF-R2 were associated with several prognostic factors for HD, including those related to the host (age, performance status) and to the tumor (disease stage, extranodal site involvement, bulky tumor, serum levels of LDH and β2-microglobulin, histology). Elevated plasma levels of TNF, TNF-R1 and TNF-R2 were also associated with several parameters reflecting an immune activation, including the presence of B symptoms, elevated serum levels of gamma globulins, alkaline phosphatase and fibrinogen, as well as peripheral monocytosis, anemia and low serum albumin levels. Additionally, elevated levels strongly predicted shorter free-from-progression survival and overall survival of the patients (110). Another study showed that TNF-α was involved in the pathogenesis of radiotherapy and chemotherapy-induced fibrosis and lung toxicity in HD which stimulate an inflammatory response and participate in the acute phase side effects by acting on mononuclear cells, neutrophils and endothelial cells (111). These results suggest the possible use of TNF and its soluble receptors as serum markers for risk assignment in HD.
A case was reported of minimal-change nephrotic syndrome associated with HD in which Hodgkin-R-S cells were immunostained for TNF-α. The case clearly demonstrated that Hodgkin-R-S produced TNF-α and in which the plasma level of TNF-α normalized after improvement of HD by chemotherapy. The production of TNF-α by Hodgkin-R-S cells might play a key role as a potential mediator of minimal change nephrotic syndrome (112).
Non-Hodgkin lymphoma (NHL) is a kind of malignant diseases of lymphoma system. Each type of NHL is closely related, involved in the malignant transformation of lymphoid cells, but with distinctive morphological, immunophenotypic, genetic, and clinical features (113). B-cell lymphomas make up the majority of cases and, of these, diffuse large B-cell lymphoma and follicular lymphomas are the two major subtypes (114). TNF-α is thought to influence lymphoma genesis through up-regulation of inflammatory and anti-apoptotic signals, possibly via the nuclear NF-kB pathway (115). In addition, TNF-α, TNF-R1 and TNF-R2 were also found to be elevated in patients with NHL and that TNF, TNF-R1 and TNF-R2 plasma levels represented valuable prognostic markers in those individuals (116,117). Studies also showed that TNF-α may cooperate with other cytokines, such as IL-6, IL-10 and IL-2 in vivo to increase NHL cell proliferation (118).
However, inhibiting TNF-α in therapy must be paid some especial attentions. Lymphoproliferative disorders have been reported in association with immunosuppression. Different types of lymphoma have been reported in patients with rheumatoid arthritis treated with anti-TNF-α, mainly large B and HD (119-121). Adams et al. reported two cases of aggressive cutaneous lymphomas after anti-TNF-α therapy with rapid onset and a fulminant clinical course. Both cases were T-cell lymphomas: Sezary syndrome and system anaplastic large cell lymphoma (AITL) with subcutaneous involvement (122). Vieites et al. presented a case of a male with chronic ulcerative colitis who secondarily developed into a cutaneous Hodgkin-type lymphoproliferative lesion associated with immunodeficiency after 6 months of treatment with anti-TNF-α (123). All these imply that further understanding of the complex mechanism in the lymphoma will be needed and can help clinicians determine in which patients these agents have a favorable risk-benefit ratio.
Conclusions
TNF-α was involved in a spectrum of physiological processes that control inflammation, anti-tumor responses and homeostasis through two receptors, TNF-R1 and TNF-R2. The role of TNF-α in cancer therapy is debated. A large body of evidence supports TNF’s antineoplastic activity while some pre-clinical findings suggest that TNF may promote cancer development and progression. In hematological diseases, TNF-α has been shown to be a bifunctional regulator of the growth of hematopoietic stem and progenitor cells. Even though some anti- TNF-α drugs have been used in clinical therapy, serious side effects have been reported, such as lymphoproliferative disorders. Therefore, inhibiting TNF-α in therapy must be paid some especial attentions. Effective targeted drugs may be expected to solve this problem. What’s more, the specific function and mechanism of TNF-α are needed to be elucidated.
Acknowledgements
Funding: This work was supported by grants from the Tai Shan Scholar Foundation, National Natural Science Foundation of China (No. 81070407, No. 81170515, No. 81070422), Outstanding Young Scientist Research Award Foundation of Shandong Province (BS2009SW014), and Independent Innovation Foundation of Shandong University (2009TS063).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Bruce-Keller AJ, Geddes JW, Knapp PE, et al. Anti-death properties of TNF against metabolic poisoning: mitochondrial stabilization by MnSOD. J Neuroimmunol 1999;93:53-71. [PubMed]
- Beutler B, Greenwald D, Hulmes JD, et al. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature 1985;316:552-4. [PubMed]
- Takeda K, Iwamoto S, Sugimoto H, et al. Identity of differentiation inducing factor and tumour necrosis factor. Nature 1986;323:338-40. [PubMed]
- Shirai T, Yamaguchi H, Ito H, et al. Cloning and expression in Escherichia coli of the gene for human tumour necrosis factor. Nature 1985;313:803-6. [PubMed]
- Takemori N, Kodaira J, Sato T, et al. Successful treatment of IBL-like T-cell lymphoma with cyclosporin A: two case reports with special reference to serum cytokine levels. Hum Cell 2000;13:35-42. [PubMed]
- Falvo JV, Tsytsykova AV, Goldfeld AE. Transcriptional control of the TNF gene. Curr Dir Autoimmun 2010;11:27-60. [PubMed]
- Giambelluca MS, Rollet-Labelle E, Bertheau-Mailhot G, et al. Post-transcriptional regulation of tumour necrosis factor alpha bipsynthesis: Relevance to the pathophysiology of rheumatoid arthritis. OA Inflammation 2013;1:3.
- Bemelmans MH, van Tits LJ, Buurman WA. Tumor necrosis factor: function, release and clearance. Crit Rev Immunol 1996;16:1-11. [PubMed]
- Derouich-Guergour D, Brenier-Pinchart MP, Ambroise-Thomas P, et al. Tumour necrosis factor alpha receptors: role in the physiopathology of protozoan parasite infections. Int J Parasitol 2001;31:763-9. [PubMed]
- Chatzikyriakidou A, Georgiou I, Voulgari PV, et al. The role of tumor necrosis factor (TNF)-alpha and TNF receptor polymorphisms in susceptibility to ankylosing spondylitis. Clin Exp Rheumatol 2009;27:645-8. [PubMed]
- Miterski B, Böhringer S, Klein W, et al. Inhibitors in the NFkappaB cascade comprise prime candidate genes predisposing to multiple sclerosis, especially in selected combinations. Genes Immun 2002;3:211-9. [PubMed]
- Kammer GM, Tsokos GC. Abnormal T lymphocyte signal transduction in systemic lupus erythematosus. Curr Dir Autoimmun 2002;5:131-50. [PubMed]
- Levine A, Shamir R, Wine E, et al. TNF promoter polymorphisms and modulation of growth retardation and disease severity in pediatric Crohn’s disease. Am J Gastroenterol 2005;100:1598-604. [PubMed]
- Fehrenbacher N, Gyrd-Hansen M, Poulsen B, et al. Sensitization to the lysosomal cell death pathway upon immortalization and transformation. Cancer Res 2004;64:5301-10. [PubMed]
- Naredi PL, Lindnér PG, Holmberg SB, et al. The effects of tumour necrosis factor alpha on the vascular bed and blood flow in an experimental rat hepatoma. Int J Cancer 1993;54:645-9. [PubMed]
- Vujanovic NL. Role of TNF family ligands in antitumor activity of natural killer cells. Int Rev Immunol 2001;20:415-37. [PubMed]
- Mocellin S, Provenzano M, Lise M, et al. Increased TIA-1 gene expression in the tumor microenvironment after locoregional administration of tumor necrosis factor-alpha to patients with soft tissue limb sarcoma. Int J Cancer 2003;107:317-22. [PubMed]
- Tselepis C, Perry I, Dawson C, et al. Tumour necrosis factor-alpha in Barrett’s oesophagus: a potential novel mechanism of action. Oncogene 2002;21:6071-81. [PubMed]
- Kitakata H, Nemoto-Sasaki Y, Takahashi Y, et al. Essential roles of tumor necrosis factor receptor p55 in liver metastasis of intrasplenic administration of colon 26 cells. Cancer Res 2002;62:6682-7. [PubMed]
- Mocellin S, Rossi CR, Pilati P, et al. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev 2005;16:35-53. [PubMed]
- Eissner G, Kolch W, Scheurich P. Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev 2004;15:353-66. [PubMed]
- Mitoma H, Horiuchi T, Tsukamoto H. Binding activities of infliximab and etanercept to transmembrane tumor necrosis factor-alpha. Gastroenterology 2004;126;934-5; author reply 935-6. [PubMed]
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol 2003;3:745-56. [PubMed]
- Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science 2002;296:1634-5. [PubMed]
- Grell M. Tumor necrosis factor (TNF) receptors in cellular signaling of soluble and membrane-expressed TNF. J Inflamm 1995-1996;47:8-17. [PubMed]
- Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell 2002;109 Suppl:S81-96. [PubMed]
- Rothe M, Wong SC, Henzel WJ, et al. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell 1994;78:681-92. [PubMed]
- Rothe M, Pan MG, Henzel WJ, et al. The TNFR2-TRAF signaling complex contains two novel proteins related to baculoviral inhibitor of apoptosis proteins. Cell 1995;83:1243-52. [PubMed]
- Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature 2002;416:345-7. [PubMed]
- Kim EY, Priatel JJ, Teh SJ, et al. TNF receptor type 2 (p75) functions as a costimulator for antigen-driven T cell responses in vivo. J Immunol 2006;176:1026-35. [PubMed]
- Theiss AL, Simmons JG, Jobin C, et al. Tumor necrosis factor (TNF) alpha increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem 2005;280:36099-109. [PubMed]
- Zhao X, Mohaupt M, Jiang J, et al. Tumor necrosis factor receptor 2-mediated tumor suppression is nitric oxide dependent and involves angiostasis. Cancer Res 2007;67:4443-50. [PubMed]
- Newman K, Maness-Harris L, El-Hemaidi I, et al. Revisiting use of growth factors in myelodysplastic syndromes. Asian Pac J Cancer Prev 2012;13:1081-91. [PubMed]
- Bachegowda L, Gligich O, Mantzaris I, et al. Signal transduction inhibitors in treatment of myelodysplastic syndromes. J Hematol Oncol 2013;6:50. [PubMed]
- Degliantoni G, Murphy M, Kobayashi M, et al. Natural killer (NK) cell-derived hematopoietic colony-inhibiting activity and NK cytotoxic factor. Relationship with tumor necrosis factor and synergism with immune interferon. J Exp Med 1985;162:1512-30. [PubMed]
- Jacobsen FW, Rothe M, Rusten L, et al. Role of the 75-kDa tumor necrosis factor receptor: inhibition of early hematopoiesis. Proc Natl Acad Sci U S A 1994;91:10695-9. [PubMed]
- Caux C, Saeland S, Favre C, et al. Tumor necrosis factor-alpha strongly potentiates interleukin-3 and granulocyte-macrophage colony-stimulating factor-induced proliferation of human CD34+ hematopoietic progenitor cells. Blood 1990;75:2292-8. [PubMed]
- Jacobsen SE, Ruscetti FW, Dubois CM, et al. Tumor necrosis factor alpha directly and indirectly regulates hematopoietic progenitor cell proliferation: role of colony-stimulating factor receptor modulation. J Exp Med 1992;175:1759-72. [PubMed]
- Caux C, Vanbervliet B, Massacrier C, et al. Interleukin-3 cooperates with tumor necrosis factor alpha for the development of human dendritic/Langerhans cells from cord blood CD34+ hematopoietic progenitor cells. Blood 1996;87:2376-85. [PubMed]
- Mizrahi K, Stein J, Yaniv I, et al. TNF-alpha has tropic rather than apoptotic activity in human hematopoietic progenitors: involvement of TNF receptor-1 and caspase-8. Stem Cells 2013;31:156-66. [PubMed]
- Pearl-Yafe M, Mizrahi K, Stein J, et al. Tumor necrosis factor receptors support murine hematopoietic progenitor function in the early stages of engraftment. Stem Cells 2010;28:1270-80. [PubMed]
- Petrides PE, Dittmann KH. How do normal and leukemic white blood cells egress from the bone marrow? Morphological facts and biochemical riddles. Blut 1990;61:3-13. [PubMed]
- Tsimberidou AM, Giles FJ. TNF-alpha targeted therapeutic approaches in patients with hematologic malignancies. Expert Rev Anticancer Ther 2002;2:277-86. [PubMed]
- Raza A, Mundle S, Shetty V, et al. A paradigm shift in myelodysplastic syndromes. Leukemia 1996;10:1648-52. [PubMed]
- Möller B, Villiger PM. Inhibition of IL-1, IL-6, and TNF-alpha in immune-mediated inflammatory diseases. Springer Semin Immunopathol 2006;27:391-408. [PubMed]
- Sugiyama H, Inoue K, Ogawa H, et al. The expression of IL-6 and its related genes in acute leukemia. Leuk Lymphoma 1996;21:49-52. [PubMed]
- Beauchemin V, Villeneuve L, Rodriguez-Cimadevilla JC, et al. Interleukin-6 production by the blast cells of acute myeloblastic leukemia: regulation by endogenous interleukin-1 and biological implications. J Cell Physiol 1991;148:353-61. [PubMed]
- Reuter S, Gupta SC, Chaturvedi MM, et al. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 2010;49:1603-16. [PubMed]
- Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer 2006;42:745-50. [PubMed]
- Sanchez-Correa B, Bergua JM, Campos C, et al. Cytokine profiles in acute myeloid leukemia patients at diagnosis: survival is inversely correlated with IL-6 and directly correlated with IL-10 levels. Cytokine 2013;61:885-91. [PubMed]
- Tsimberidou AM, Estey E, Wen S, et al. The prognostic significance of cytokine levels in newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndromes. Cancer 2008;113:1605-13. [PubMed]
- Aguayo A, Kantarjian H, Manshouri T, et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood 2000;96:2240-5. [PubMed]
- Guilloton F, de Thonel A, Jean C, et al. TNFalpha stimulates NKG2D-mediated lytic activity of acute myeloid leukemic cells. Leukemia 2005;19:2206-14. [PubMed]
- Ismair MG, Ries C, Lottspeich F, et al. Autocrine regulation of matrix metalloproteinase-9 gene expression and secretion by tumor necrosis factor-alpha (TNF-alpha) in NB4 leukemic cells: specific involvement of TNF receptor type 1. Leukemia 1998;12:1136-43. [PubMed]
- Cimino G, Amadori S, Cava MC, et al. Serum interleukin-2 (IL-2), soluble IL-2 receptors and tumor necrosis factor-alfa levels are significantly increased in acute myeloid leukemia patients. Leukemia 1991;5:32-5. [PubMed]
- Kurzrock R, Kantarjian H, Wetzler M, et al. Ubiquitous expression of cytokines in diverse leukemias of lymphoid and myeloid lineage. Exp Hematol 1993;21:80-5. [PubMed]
- Miyazaki Y. Revised international prognostic scoring system (IPSS-R) for myelodysplastic syndromes. Rinsho Ketsueki 2013;54:545-51. [PubMed]
- Greenberg PL. Apoptosis and its role in the myelodysplastic syndromes: implications for disease natural history and treatment. Leuk Res 1998;22:1123-36. [PubMed]
- Gersuk GM, Beckham C, Loken MR, et al. A role for tumour necrosis factor-alpha, Fas and Fas-Ligand in marrow failure associated with myelodysplastic syndrome. Br J Haematol 1998;103:176-88. [PubMed]
- Dar S, Mundle S, Andric T, et al. Biological characteristics of myelodysplastic syndrome patients who demonstrated high versus no intramedullary apoptosis. Eur J Haematol 1999;62:90-4. [PubMed]
- Sawanobori M, Yamaguchi S, Hasegawa M, et al. Expression of TNF receptors and related signaling molecules in the bone marrow from patients with myelodysplastic syndromes. Leuk Res 2003;27:583-91. [PubMed]
- Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell 1995;81:495-504. [PubMed]
- Grzybowska-Izydorczyk O, Smolewski P. The role of the inhibitor of apoptosis protein (IAP) family in hematological malignancies. Postepy Hig Med Dosw (Online) 2008;62:55-63. [PubMed]
- Parnes A, Nikiforow S, Berliner N, et al. Single nucleotide polymorphisms in the human TNF gene are associated with anaemia and neutropenia in a cohort of patients with de novo myelodysplastic syndrome. Br J Haematol 2010;150:700-1. [PubMed]
- Ishibashi M, Tamura H, Ogata K. Disease progression mechanism in myelodysplastic syndromes: insight into the role of the microenvironment. Leuk Res 2011;35:1449-52. [PubMed]
- Stirewalt DL, Mhyre AJ, Marcondes M, et al. Tumour necrosis factor-induced gene expression in human marrow stroma: clues to the pathophysiology of MDS? Br J Haematol 2008;140:444-53. [PubMed]
- Deeg HJ, Gotlib J, Beckham C, et al. Soluble TNF receptor fusion protein (etanercept) for the treatment of myelodysplastic syndrome: a pilot study. Leukemia 2002;16:162-4. [PubMed]
- Raza A, Candoni A, Khan U, et al. Remicade as TNF suppressor in patients with myelodysplastic syndromes. Leuk Lymphoma 2004;45:2099-104. [PubMed]
- Scott BL, Ramakrishnan A, Fosdal M, et al. Anti-thymocyte globulin plus etanercept as therapy for myelodysplastic syndromes (MDS): a phase II study. Br J Haematol 2010;149:706-10. [PubMed]
- D’Andrea AD, Grompe M. Molecular biology of Fanconi anemia: implications for diagnosis and therapy. Blood 1997;90:1725-36. [PubMed]
- Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood 1995;86:2856-62. [PubMed]
- Joenje H, Patel KJ. The emerging genetic and molecular basis of Fanconi anaemia. Nat Rev Genet 2001;2:446-57. [PubMed]
- Meetei AR, de Winter JP, Medhurst AL, et al. A novel ubiquitin ligase is deficient in Fanconi anemia. Nat Genet 2003;35:165-70. [PubMed]
- Levitus M, Rooimans MA, Steltenpool J, et al. Heterogeneity in Fanconi anemia: evidence for 2 new genetic subtypes. Blood 2004;103:2498-503. [PubMed]
- Carreau M, Gan OI, Liu L, et al. Bone marrow failure in the Fanconi anemia group C mouse model after DNA damage. Blood 1998;91:2737-44. [PubMed]
- Li X, Yang Y, Yuan J, et al. Continuous in vivo infusion of interferon-gamma (IFN-gamma) preferentially reduces myeloid progenitor numbers and enhances engraftment of syngeneic wild-type cells in Fancc-/- mice. Blood 2004;104:1204-9. [PubMed]
- Si Y, Ciccone S, Yang FC, et al. Continuous in vivo infusion of interferon-gamma (IFN-gamma) enhances engraftment of syngeneic wild-type cells in Fanca-/- and Fancg-/- mice. Blood 2006;108:4283-7. [PubMed]
- Dufour C, Corcione A, Svahn J, et al. TNF-alpha and IFN-gamma are overexpressed in the bone marrow of Fanconi anemia patients and TNF-alpha suppresses erythropoiesis in vitro. Blood 2003;102:2053-9. [PubMed]
- Schultz JC, Shahidi NT. Tumor necrosis factor-alpha overproduction in Fanconi’s anemia. Am J Hematol 1993;42:196-201. [PubMed]
- Rosselli F, Sanceau J, Gluckman E, et al. Abnormal lymphokine production: a novel feature of the genetic disease Fanconi anemia. II. In vitro and in vivo spontaneous overproduction of tumor necrosis factor alpha. Blood 1994;83:1216-25. [PubMed]
- Dealtry GB, Naylor MS, Fiers W, et al. DNA fragmentation and cytotoxicity caused by tumor necrosis factor is enhanced by interferon-gamma. Eur J Immunol 1987;17:689-93. [PubMed]
- Rubin BY, Smith LJ, Hellermann GR, et al. Correlation between the anticellular and DNA fragmenting activities of tumor necrosis factor. Cancer Res 1988;48:6006-10. [PubMed]
- Dutrillaux B, Aurias A, Dutrillaux AM, et al. The cell cycle of lymphocytes in Fanconi anemia. Hum Genet 1982;62:327-32. [PubMed]
- Weksberg R, Buchwald M, Sargent P, et al. Specific cellular defects in patients with Fanconi anemia. J Cell Physiol 1979;101:311-23. [PubMed]
- Heim RA, Lench NJ, Swift M. Heterozygous manifestations in four autosomal recessive human cancer-prone syndromes: ataxia telangiectasia, xeroderma pigmentosum, Fanconi anemia, and Bloom syndrome. Mutat Res 1992;284:25-36. [PubMed]
- Bagnara GP, Bonsi L, Strippoli P, et al. Production of interleukin 6, leukemia inhibitory factor and granulocyte-macrophage colony stimulating factor by peripheral blood mononuclear cells in Fanconi’s anemia. Stem Cells 1993;11 Suppl 2:137-43. [PubMed]
- Sejas DP, Rani R, Qiu Y, et al. Inflammatory reactive oxygen species-mediated hemopoietic suppression in Fancc-deficient mice. J Immunol 2007;178:5277-87. [PubMed]
- Li J, Sejas DP, Zhang X, et al. TNF-alpha induces leukemic clonal evolution ex vivo in Fanconi anemia group C murine stem cells. J Clin Invest 2007;117:3283-95. [PubMed]
- Mehta PA, Svahn J, Davies SM, et al. Etanercept treatment in Fanconi anaemia; combined US and Italian experience. Br J Haematol 2012;158:809-11. [PubMed]
- Shapiro-Shelef M, Calame K. Plasma cell differentiation and multiple myeloma. Curr Opin Immunol 2004;16:226-34. [PubMed]
- Jurisić V, Colović M. Correlation of sera TNF-alpha with percentage of bone marrow plasma cells, LDH, beta2-microglobulin, and clinical stage in multiple myeloma. Med Oncol 2002;19:133-9. [PubMed]
- Sánchez-Beato M, Sánchez-Aguilera A, Piris MA. Cell cycle deregulation in B-cell lymphomas. Blood 2003;101:1220-35. [PubMed]
- Jöhrer K, Janke K, Krugmann J, et al. Transendothelial migration of myeloma cells is increased by tumor necrosis factor (TNF)-alpha via TNF receptor 2 and autocrine up-regulation of MCP-1. Clin Cancer Res 2004;10:1901-10. [PubMed]
- Bharti AC, Shishodia S, Reuben JM, et al. Nuclear factor-kappaB and STAT3 are constitutively active in CD138+ cells derived from multiple myeloma patients, and suppression of these transcription factors leads to apoptosis. Blood 2004;103:3175-84. [PubMed]
- Li ZW, Chen H, Campbell RA, et al. NF-kappaB in the pathogenesis and treatment of multiple myeloma. Curr Opin Hematol 2008;15:391-9. [PubMed]
- Wajant H. Death receptors. Essays Biochem 2003;39:53-71. [PubMed]
- Jourdan M, Tarte K, Legouffe E, et al. Tumor necrosis factor is a survival and proliferation factor for human myeloma cells. Eur Cytokine Netw 1999;10:65-70. [PubMed]
- Dmoszyńska A, Bojarska-Junak A, Domański D, et al. Production of proangiogenic cytokines during thalidomide treatment of multiple myeloma. Leuk Lymphoma 2002;43:401-6. [PubMed]
- Landgren O, Weiss BM. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: support for genetic factors in pathogenesis. Leukemia 2009;23:1691-7. [PubMed]
- Davies FE, Rollinson SJ, Rawstron AC, et al. High-producer haplotypes of tumor necrosis factor alpha and lymphotoxin alpha are associated with an increased risk of myeloma and have an improved progression-free survival after treatment. J Clin Oncol 2000;18:2843-51. [PubMed]
- Martino A, Buda G, Maggini V, et al. Could age modify the effect of genetic variants in IL6 and TNF-alpha genes in multiple myeloma? Leuk Res 2012;36:594-7. [PubMed]
- Barlogie B, Zangari T, Spencer A, et al. Thalidomide in the management of multiple myeloma. Semin Hematol 2001;38:250-9. [PubMed]
- D’Amato RJ, Loughnan MS, Flynn E, et al. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A 1994;91:4082-5. [PubMed]
- Vacca A, Ribatti D, Presta M, et al. Bone marrow neovascularization, plasma cell angiogenic potential, and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood 1999;93:3064-73. [PubMed]
- Kast RE. Evidence of a mechanism by which etanercept increased TNF-alpha in multiple myeloma: new insights into the biology of TNF-alpha giving new treatment opportunities--the role of bupropion. Leuk Res 2005;29:1459-63. [PubMed]
- Shareef MS, Munro LR, Owen RG, et al. Progression of IgA gammopathy to myeloma following infliximab treatment for pyoderma gangrenosum. Clin Exp Dermatol 2012;37:146-8. [PubMed]
- Marafioti T, Hummel M, Foss HD, et al. Hodgkin and reed-sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood 2000;95:1443-50. [PubMed]
- Warzocha K, Bienvenu J, Coiffier B, et al. Mechanisms of action of the tumor necrosis factor and lymphotoxin ligand-receptor system. Eur Cytokine Netw 1995;6:83-96. [PubMed]
- Le Hir M, Bluethmann H, Kosco-Vilbois MH, et al. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling. J Exp Med 1996;183:2367-72. [PubMed]
- Warzocha K, Bienvenu J, Ribeiro P, et al. Plasma levels of tumour necrosis factor and its soluble receptors correlate with clinical features and outcome of Hodgkin's disease patients. Br J Cancer 1998;77:2357-62. [PubMed]
- Villani F, Busia A, Villani M, et al. Serum cytokine in response to chemo-radiotherapy for Hodgkin’s disease. Tumori 2008;94:803-8. [PubMed]
- Nakayama S, Yokote T, Kobayashi K, et al. Minimal-change nephrotic syndrome preceding Hodgkin lymphoma by 5 years with expression of tumor necrosis factor alpha in Hodgkin-Reed-Sternberg cells. Hum Pathol 2010;41:1196-9. [PubMed]
- Morton LM, Purdue MP, Zheng T, et al. Risk of non-Hodgkin lymphoma associated with germline variation in genes that regulate the cell cycle, apoptosis, and lymphocyte development. Cancer Epidemiol Biomarkers Prev 2009;18:1259-70. [PubMed]
- Skibola CF, Curry JD, Nieters A. Genetic susceptibility to lymphoma. Haematologica 2007;92:960-9. [PubMed]
- Purdue MP, Lan Q, Kricker A, et al. Polymorphisms in immune function genes and risk of non-Hodgkin lymphoma: findings from the New South Wales non-Hodgkin Lymphoma Study. Carcinogenesis 2007;28:704-12. [PubMed]
- Salles G, Bienvenu J, Bastion Y, et al. Elevated circulating levels of TNFalpha and its p55 soluble receptor are associated with an adverse prognosis in lymphoma patients. Br J Haematol 1996;93:352-9. [PubMed]
- Warzocha K, Salles G, Bienvenu J, et al. Tumor necrosis factor ligand-receptor system can predict treatment outcome in lymphoma patients. J Clin Oncol 1997;15:499-508. [PubMed]
- Voorzanger N, Touitou R, Garcia E, et al. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin's lymphoma cells and act as cooperative growth factors. Cancer Res 1996;56:5499-505. [PubMed]
- Paul C, Le Tourneau A, Cayuela JM, et al. Epstein-Barr virus-associated lymphoproliferative disease during methotrexate therapy for psoriasis. Arch Dermatol 1997;133:867-71. [PubMed]
- Wolfe F, Michaud K. The effect of methotrexate and anti-tumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 person-years of observation. Arthritis Rheum 2007;56:1433-9. [PubMed]
- Kumar S, Kingma DW, Weiss WB, et al. Primary cutaneous Hodgkin’s disease with evolution to systemic disease. Association with the Epstein-Barr virus. Am J Surg Pathol 1996;20:754-9. [PubMed]
- Adams AE, Zwicker J, Curiel C, et al. Aggressive cutaneous T-cell lymphomas after TNFalpha blockade. J Am Acad Dermatol 2004;51:660-2. [PubMed]
- Vieites B, Avila R, Biscuola M, et al. Cutaneous Hodgkin-type lymphoproliferative lesion associated with immunomodulatory therapy for ulcerative colitis. J Cutan Pathol 2011;38:443-7. [PubMed]
Cite this article as: Tian T, Wang M, Ma D. TNF-α, a good or bad factor in hematological diseases? Stem Cell Investig 2014;1:12.


