Recent advances and novel agents for FLT3 mutated acute myeloid leukemia
Introduction
Acute myeloid leukemia (AML) accounts for approximately 30% of leukemia’s but >40% of leukemia-related deaths (1). AML is highly diverse and cytogenetic analysis of metaphase cells reveals that approximately 40-50% of patients with de novo AML have a normal karyotype. The clinical outcomes for these AML patients with a normal karyotype are heterogeneous. Mutations in key genes involved in AML pathogenesis lead to large inter patient variability in prognosis and outcomes (2-4). Some of these mutations like the internal tandem duplication (ITD) in the FMS-like tyrosine kinase receptor-3 (FLT3) gene, partial tandem duplication in the mixed lineage leukemia gene (5), Wilm’s tumor gene mutations (6) and overexpression of the BAALC gene (7) are associated with poor prognosis in AML (8). On the other hand, CCAAT/enhancer-binding protein α (CEBPA) and nucleophosmin 1 (NPM1) mutations (in FLT3-ITD-negative patients) have been associated with improved prognosis (8,9). In this review, we discussed FLT3 mutations and the latest advances reported in 2012-2013 regarding emerging approaches using FLT3 inhibitors for the treatment of AML.
FLT3 receptor
Mutations in FLT3 are one of the most common and clinically relevant mutations in patients with AML (Figure 1). FLT3 is a trans-membrane tyrosine kinase receptor expressed on the leukemic blasts of 70-100% of patients with AML (10,11). FLT3 dimerizes and undergoes a conformational change when it binds to the FLT3 ligand (FL). This opens up the activation loop (AL) and reveals the ATP-binding pocket. The receptor becomes auto phosphorylated and stimulates cell proliferation and inhibits apoptosis through the activation of a signaling cascade including PI3K/Akt, MAPK/ERK, and STATs (12). The FLT3 receptor is restricted primarily to hematopoietic and neural tissues 21 but the ligand is ubiquitously expressed (13,14).
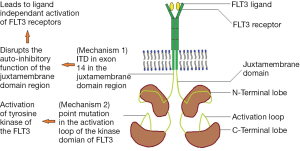
FLT3 mutations
The exact frequency of FLT3 varies with age with mutations being present in approximately 20 percent of patients with cytogenetically normal AML (15,16). There are two major types of FLT3 mutations. The most common is an ITD on exon 14 of the FLT3 gene, which varies from 3 to >400 base pairs. It is typically in frame and occurs in the juxta-membrane region of the receptor (17). This ITD disrupts the auto inhibitory function of the juxta-membrane domain and results in ligand-independent activation of the FLT3 receptor. This leads to a proliferative signal via activation of its downstream effectors (18,19), and produces growth factor-independent proliferation in leukemia cell lines and a fatal myeloproliferative syndrome in murine models. Thus, the ITD leads to gain of function mainly by inducing hyper-responsivity of the FLT3 (2,3) receptor to FL rather than through auto-activation of the receptor (20). Alternatively, point mutations in the activating loop of the kinase domain of FLT3 may result in tyrosine kinase activation of FLT3 in 5 to 10 percent of patients (21,22). The most common of these activating mutations is the aspartate 835 (D835) mutation, which occurs in approximately 7% of patients with AML (22,23).
The impact of FLT3-ITD on prognosis and long term outcomes has been studied in several analysis and trials (24-29). Patients with FLT3-ITD AML achieve complete remission rates comparable to those of patients with wild-type disease, but have significantly higher rates of relapse and shorter durations of disease-free and overall survival (OS). In contrast, the mutations of the tyrosine kinase domain of FLT3 do not appear to be associated with the same poor outcome as FLT3/ITD (21). Recently it has also been suggested both the length (30) and location (31) of ITD insertions can negatively affect the prognostic outcome of patients, as can the absence of the wild-type allele in FLT3-ITD-positive patients (32), whereas stem cell transplantation can improve patient survival.
FLT3-ITD-expressing murine and human myeloid cell lines are resistant to cytosine arabinoside. Additional, in vitro evidence also suggests that FLT3-ITD may contribute to drug resistance (33). This has led to intensive research to identify therapeutic inhibitors of FLT3. Several of these agents are currently in clinical trials and have shown promising anti-leukemic activity. The presence of FLT3-ITD, however, is not the only relevant factor in determining patient outcome. AML is a complex disease characterized by multiple genetic abnormalities that likely interact with one another. Understanding how the myriad signaling pathways underlying the pathogenesis of AML interact with one another will be critical in determining how particular genetic abnormalities affect prognosis and response to therapy.
FLT3 inhibitors in clinical trials
A variety of novel agents targeting FLT3 have been developed and reported in 2012-2013. These are undergoing clinical trials (Table 1).
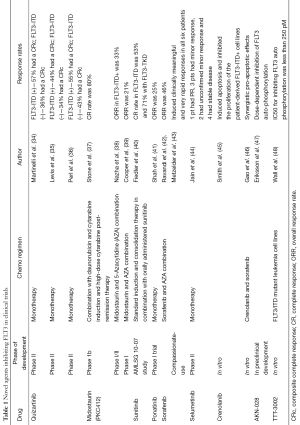
Full table
Quizartinib
Li et al. recently reported at ASH 2012 on the absorption, metabolism and excretion of quizartinib in six healthy volunteers (49). All volunteers received a single oral dose of 60 mg quizartinib as a solution. Quizartinib was extensively metabolized mainly by phase I biotransformation, with the metabolites excreted primarily in feces. It was well tolerated. Two out of six volunteers experienced adverse events. Grade 1 dry skin in one subject was considered to be unrelated to study treatment, and grade 1 diarrhea in one subject was considered to be possibly related to treatment.
Quizartinib has also been studied in a phase II monotherapy trial in patients with FLT3-ITD positive or negative relapsed/refractory AML after second-line chemotherapy or hematopoietic stem cell transplantation. This was an efficacy and safety analysis. The study comprising of two cohorts has been completed but only preliminary results are available. It has not yet been reported in its entirety. They included 333 patients in two cohorts. All patients received quizartinib at a starting dose of 90 mg/day (females) or 135 mg/day (males and one female), and were treated continuously during 28-day cycles. The two cohorts were analyzed separately. The composite complete remission (CRc) rate was calculated which included complete remission (CR), complete remission with incomplete platelet recovery (CRp), and complete remission with incomplete hematologic recovery (CRi).
Cohort 1 included patients aged ≥60 years with AML relapsed in <1 year or refractory to 1st-line chemotherapy. A total of 154 patients were included in this cohort. These patients included 110 (71%) who were FLT3 ITD (+), 44 (29%) who were FLT3-ITD (–). Of 110 FLT3-ITD (+) patients, 63 (57%) had a CRc and 16 of 44 FLT3-ITD (–) patients had a CRc. Median OS in FLT3-ITD (+) patients was 25.3 weeks and 16 out of 110 survived >52 weeks. The median age of patients surviving >52 weeks was 69.5 years. Median OS in FLT3-ITD (–) patients was 19.1 weeks and six out of 44 FLT3-ITD (–) patients (14%) survived >52 weeks. The median age of these patients was 70.0 years (34).
At ASH 2012 Cortes et al. had reported the preliminary results of this trial in 134 patients. The most common (≥20%) treatment-related adverse events (AEs) were nausea, fatigue, anemia, QT interval prolongation, diarrhea, vomiting, dysgeusia and febrile neutropenia (50).
Cohort 2 included 137 patients aged more than/equal to 18 years with AML relapsed or refractory to 2nd-line, salvage chemotherapy or relapsed after hematopoietic stem cell transplantation (HSCT). Ninety-nine (72%) were FLT3-ITD (+) and 38 (28%) were FLT3-ITD negative. The FLT3-ITD (+) patients had a median age of 50 years and the FLT3-ITD (–) patients had a median age of 55 years. For FLT3-ITD (+) patients the CRc rate was 44% (4% CR, 0 CRp and 40% CRi), median duration of response was 11.3 weeks and median OS was 23.1 weeks. Patients who were refractory to their last AML therapy had a 47% CRc with quizartinib. FLT3-ITD (–) patients had a CRc rate of 34% (3% CR, 3% CRp and 29% CRi), a median duration of response of 5.0 weeks and median OS of 25.6 weeks. Patients who were refractory to their last AML therapy had a 31% CRc with quizartinib. The most common (≥20%) treatment-related adverse events (AEs) were nausea, anemia, QT interval prolongation, vomiting, febrile neutropenia, diarrhea and fatigue. Thus, this phase II study confirmed the activity of quizartinib monotherapy in FLT3-ITD (+) and FLT3-ITD (–) AML patients relapsed/refractory to 2nd line therapy or HSCT (35).
At ASCO 2013, Cortes et al. reported on the response rates and bridging to hematopoietic stem cell transplantation with quizartinib (AC220) in patients with FLT3-ITD positive or negative relapsed/refractory AML after second-line chemotherapy or previous bone marrow transplant (51). They included 136 FLT3-ITD (+) and 40 FLT3-ITD (–) patients. Quizartinib was discontinued for HSCT in 47 out of 136 patients and 44/47 patients at least had a PR (2 CRp, 24 CRi, 18 PR). Among FLT3-ITD (–) patients, quizartinib was discontinued for HSCT in 14/40 and 13 out of 14 had achieved at least a PR (one CR, one CRp, seven CRi, four PR). The median OS for FLT3-ITD (+) patients with CRc prior to HSCT was 41.5 weeks and 1-year survival was 39%. If the patients were in PR prior to HSCT, median OS was 29 weeks and 1-year survival was 39%. Patients with a CRc (n=36) or PR (n=20) but no HSCT, respectively, had a median OS of 24.5 and 20.9 weeks and 1-year survival rates of 25% and 5%. For FLT3-ITD (–) patients, median OS with CRc was not reached, 1-year survival rate was 78%. The median OS with PR was 40.7 weeks and 1-year survival rate was 50%. The authors concluded that 33% patients who had failed HSCT or salvage chemotherapy were successfully bridged to HSCT with encouraging 1-year survival.
Perl et al. reported a study of quizartinib in patients age ≥70 years with FLT3-ITD positive or negative relapsed/refractory AML (36). They reported CRc of 32 (53%) in FLT3-ITD (+) (60 patients). In FLT3-ITD (–) (23 patients), CRc was 10 (43%) (two CR, one CRp, seven CRi). Interestingly, 44% FLT3-ITD (+) and 50% FLT3-ITD (–) patients refractory to prior therapy responded to quizartinib. Thus, elderly patients with chemotherapy-resistant AML had preserved high response rates and promising survival on quizartinib.
Midostaurin (PKC412)
PKC412 is another potent orally bioavailable FLT3 inhibitor. Stone and co-workers recently reported on a phase Ib trial that evaluated several doses and schedules of midostaurin in combination with daunorubicin and cytarabine induction and high-dose cytarabine post-remission therapy in newly diagnosed patients with AML (37). The discontinuation rate on the 50-mg twice-daily dose schedule was lower than 100 mg twice daily, and no grade 3/4 nausea or vomiting was seen. The CR rate for the midostaurin 50-mg twice-daily dose schedule was 80% [FLT3-wild-type: 20 of 27 (74%), FLT3-mutant: 12 of 13 (92%)]. OS probabilities of patients with FLT3-mutant AML at one and two years (0.85 and 0.62, respectively) were similar to the FLT3-wild-type population (0.78 and 0.52, respectively). They concluded that midostaurin in combination with standard chemotherapy demonstrated high complete response (CR) and OS rates in newly diagnosed younger adults with AML, and was generally well tolerated at 50 mg twice daily for 14 days. A phase III prospective trial is ongoing (CALGB 10603, NCT00651261).
At ASH 2012, Nazha et al. presented a phase I/II trial of midostaurin (PKC412) and 5-azacytidine (AZA) for the treatment of patients with refractory or relapsed AML or myelodysplastic syndrome (MDS) (38). Patients were eligible if they were more than/equal to 18 years with MDS, chronic myelomonocytic leukemia or AML, who failed prior therapies. In phase I, patients were included regardless of their FLT3 mutation status; in phase II, only patients with FLT3 mutations were included. Five patients had FLT3-ITD and one patient had FLT3-D835 mutation only. All patients received a daily dosage of 5-AZA at 75 mg/m2 subcutaneously or intravenously for seven days on days 1-7 of each cycle and midostaurin at 25 mg (cohort 1) and 50 mg (cohort 2; target dose) orally and twice daily for 14 days (Days 8-21) of each cycle. Patients were planned to receive up to 12 cycles if benefit from treatment. A total of 20 patients were included in the study. Thirteen patients were enrolled during phase I and six patients during phase II. Overall response rate (ORR) in phase I was 3/13 (21%) and in phase II was 2/6 (33%). For patients with FLT3-ITD mutations, 1/4 (25%) achieved Cri during phase I. In total, the ORR among patients with FLT3-ITD mutations was 3/9 (33%). All toxicities were grade 1 and 2 with no difference between the two dose schedules of midostaurin.
Cooper and colleagues reported a similar phase I study at ASCO 2013 in relapsed and elderly AML patients (39). Over a 28-day cycle in untreated elderly/relapsed AML, AZA 75 mg/m2 was given for seven days and then started on gradual increasing dose of midostaurin to 75 mg BID. They included 17 patients with median age of 73. All patients were FLT3 negative. There were no dose limiting toxicities. Fourteen out of seventeen patients were evaluable and included two CR’s and one PR. Thus, the combination of midostaurin/AZA is safe and well tolerated.
Sunitinib
Sunitinib is a multi-targeted FLT3 inhibitor approved for the treatment of advanced/metastatic renal cancer, advanced pancreatic neuro-endocrine tumors and metastatic/unresectable malignant GIST after failure of imatinib (1,14,52). Sunitinib has been evaluated in refractory AML as single agent treatment resulting in transient blast count reduction and in several cases of partial response in AML with activating FLT3 mutations. Fiedler et al. reported at ASH 2012 the results of the combination of sunitinib and intensive chemotherapy in patients with AML and activating FLT3 mutations (40). These are the results of the AMLSG 10-07 study. The study aimed at evaluating the feasibility of a standard induction and consolidation therapy in combination with orally administered sunitinib in elderly AML patients with activating FLT3 mutations. Patients aged 60 years or higher with AML with activating FLT3 mutations (FLT3-ITD, FLT3-TKD) and fit for intensive chemotherapy were eligible. A total of 22 patients were enrolled. The median age was 70 years and the type of AML was de novo in 16 patients, secondary AML in one patient and therapy related AML in four pts. Fifteen patients had a FLT3-ITD (68%) and seven had a FLT3-TKD (32%) mutation. Response to induction therapy in all patients was CR in 13 patients (59%), partial remission in one patient (4.5%), and refractory disease in five patients (23%), death in three patients (13.5%). CR rate in AML with FLT3-ITD was 53% (8/15) and 71% (5/7) in those with FLT3-TKD. All 13 patients achieving CR received repetitive cycles of high-dose cytarabine consolidation therapy and seven proceeded to single agent sunitinib maintenance therapy [median 11 months (range, 1-24 months)]. In these patients relapse occurred in ten, one patient died due to severe colitis during consolidation therapy and two patients are in sustained CR. Median survival was of 18.8 months and a 2-year survival of 36% (95% CI, 19-70%). Median relapse-free survival was 11 months.
Single agent trials with sunitinib utilize higher dose than used in this study and have been reported in the past.
Ponatinib
Recent pharmacological research has revealed ponatinib that is a multiple tyrosine kinase inhibitor and potent pan-BCR-ABL inhibitor. In preclinical models, ponatinib also inhibits the FLT3/ITD mutant prevalent in AML with potency similar to that of BCR-ABL (53). A cohort of patients with relapsed refractory AML were enrolled in a phase I trial of ponatinib (45 mg once daily) to assess safety and preliminary efficacy in this population. Shah et al. recently published the results (41). Twelve patients were enrolled with a median age of 49 years. All patients had a history of a FLT3 mutation. Mutational analysis in a central laboratory confirmed the presence of FLT3-ITD in seven patients. The ORR was 3/12 (25%). Two patients achieved a complete remission with incomplete blood count recovery and one patient experienced partial remission. These three responders carried FLT3-ITD mutations and were all FLT3 inhibitor-naïve. The duration of ponatinib treatment in these patients was 3-6 months. Nine patients experienced at least one treatment-related AE. All patients in the study discontinued treatment for various reasons including death, adverse event, investigator decision or disease progression.
Sorafenib
Ravandi et al. examined the efficacy of combining sorafenib and AZA in patients with AML and the FLT3-ITD mutation (42). Patients received AZA 75 mg/m2 IV daily for seven days and sorafenib 400 mg PO twice daily continuously and cycles were repeated at approximately one month intervals. They enrolled forty-three AML patients with a median age of 64 years but only 37 patients were evaluable. The FLT-3-ITD mutation was detected in 40 (93%) patients. The ORR was 46%, including ten (27%) CRi, six (16%) CR and one (3%) PR. They reported a median time to achieve CR/CRi as two cycles and the median duration of CR/CRi was 2.3 months. Thus, they concluded that the combination of AZA and sorafenib is effective for patients with relapsed AML and the FLT-3-ITD mutation.
Prior to this several investigators have reported anecdotal evidence of the use of sorafenib in FLT3 mutated patients (54). Metzelder and colleagues reported the compassionate-use results for the treatment of relapsed or refractory FLT3-ITD-positive AML (43). Sorafenib induced clinically meaningful and very rapid responses in all six patients treated either before (n=2), after (n=3), or both before and after (n=1) allogeneic stem cell transplantation (allo-SCT). These remissions were a bridge to allo-SCT in two of the three refractory patients. Two of the four patients who were treated after allogeneic transplant survived 216 and 221 days, respectively, whereas the other two were in ongoing complete molecular remission at the time of publication.
Selumetinib (AZD6244)
Selumetinib is an orally available MEK kinase inhibitor (55). Jain presented the results of a multi-center phase II study of selumetinib in advanced AML at ASCO 2012 (44). The dose used in this study was 100 mg twice daily. Forty-seven patients were enrolled and the median number of cycles received was one. Grade ≥3 adverse drug reactions included fatigue, dyspnea and nausea. One patient had partial response, three patients had minor response (defined as a >50% decrease in blood and/or marrow blasts lasting >4 weeks) and two patients had unconfirmed minor response (>50% decline in marrow blasts without a follow up confirmatory biopsy). In addition, four patients had stable disease. However, no patient with FLT3 ITD or NRAS mutation responded. This data indicates that it does have anti-leukemic activity but activity against FLT3 is not proven.
Crenolanib (CP-868,596)
Crenolanib is a potent and selective type I FLT3 inhibitor. Smith and co-workers hypothesized that crenolanib may be a Type I inhibitor of FLT3 that retains activity against FLT3 mutant isoforms, including AC220-resistant FLT3 AL mutants, which are highly cross-resistant to multiple FLT3 TKIs (45). They published their results and confirmed that crenolanib is a Type I inhibitor as it bound preferentially to the phosphorylated form of ABL. It also demonstrated substantially more potent in vitro binding affinity for the compound FLT3-ITD/D835V mutant than AC220. Crenolanib induced apoptosis and inhibited the proliferation of the patient-derived FLT3-ITD+ cell lines. Thus, the authors concluded that crenolanib has a potential to be clinically active in AML patients with activating FLT3-ITD or AL mutations, and to recapture clinical response in patients with acquired AC220-resistant kinase domain mutations.
Gao et al. reported (46) on the combination of crenolanib and sorafenib in FLT3-ITD inhibitor resistant AML with FLT3 mutations at ASH 2012. They hypothesized that targeting different sites of FLT3 protein simultaneously using different types of kinase inhibitors may be effective in overcoming sorafenib resistance. They found that sorafenib-resistant cells Baf3-ITD+Res and Baf3-ITD+842/676 when exposed to sub-micro molar concentrations of crenolanib and sorafenib concomitantly for 48 hours resulted in impressive synergistic pro-apoptotic effects. This implies a high synergistic potency of Type I and Type II FLT3 kinase inhibitors, when given concomitantly and is a therapeutic rationale for a combinatorial treatment strategy with crenolanib and sorafenib of FLT-ITD inhibitor-refractory AML. Crenolanib is currently in Phase II clinical trials in relapsed/refractory AML (NCT01657682, NCT01522469).
AKN-028
AKN-028 is a FLT-3 kinase inhibitor in preclinical development. Eriksson et al. reported that AKN-028 causes dose-dependent inhibition of FLT3 auto-phosphorylation (47). AKN-028 showed cytotoxic activity in all five AML cell lines. AKN-028 triggered apoptosis in MV4-11 by activation of caspase 3. Combination studies showed synergistic activity when cytarabine or daunorubicin was added simultaneously or 24 h before AKN-028. However, the response did not correlate at all with the type of mutation. The authors also postulated that anti-leukemic effect of AKN028 might be independent of FLT3 inhibition. Taking these facts in consideration and pre-maturity of data this agent can go in several different directions in future. It is a candidate drug for clinical trials which are ongoing (NCT01573247).
TTT-3002
TTT-3002 is another novel FLT3 tyrosine kinase inhibitor which was reported for the first time at ASH 2012 (48).Utilizing human FLT3/ITD mutant leukemia cell lines the presenters showed that the half maximal inhibitory concentration (IC50) for inhibiting FLT3 auto phosphorylation was less than 250 picomolar (pM). The IC50 for TTT-3002 in inhibiting proliferation in these same FLT3/ITD expressing cells was 490-920 pM. TTT-3002 also showed potent activity when tested against a broad spectrum of known, non-ITD FLT3 activating mutations, including the most frequently occurring D835Y mutation. It was demonstrated that TTT-3002 is cytotoxic to leukemic blasts isolated from FLT3/ITD expressing AML patients, while displaying minimal toxicity against normal hematopoietic stem/progenitor cells from healthy bone marrow donors.
Variations in FL levels during the course of AML treatment
Grunwald et al. (56) reported at ACO 2013 the results of a pilot study designed to see the relationship between AML therapy and FL levels over time. Ten AML patients whose blood samples were collected weekly were enrolled. They noted four distinct patterns during the study. In patients with aplastic bone marrow (in nine patients) after chemotherapy, FL concentrations rose markedly and consistently to levels >1,000 picogram/milliliter (pg/mL). In three of four patients FL concentrations remained <500 pg/mL during induction in which leukemia was refractory to induction chemotherapy. In two patients receiving FLT3 TKI sorafenib, FL concentrations remained <500 pg/mL. In one patient receiving 5-azacitidine FL concentrations remained <100 pg/mL throughout the therapy. Thus, if there is residual leukemia as opposed to an aplastic bone marrow after chemotherapy then FL concentration does not rise dramatically in patients on targeted agents.
Conclusions and future directions
In summary, a deeper scientific understanding of AML and FLT3 mutation has led to the development of multiple novel therapeutic agents for this patient population. These are summarized on Table 1. With improved technology and molecular targeted agents, this translation of science into applied therapeutics should continue to move forward at a very rapid pace. It is foreseeable that more agents with novel mechanisms of action and targeting different pathways will be studied for leukemia therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Fröhling S, Scholl C, Gilliland DG, et al. Genetics of myeloid malignancies: pathogenetic and clinical implications. J Clin Oncol 2005;23:6285-95. [PubMed]
- Mrózek K, Marcucci G, Paschka P, et al. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood 2007;109:431-48. [PubMed]
- Mrózek K, Bloomfield CD. Chromosome aberrations, gene mutations and expression changes, and prognosis in adult acute myeloid leukemia. Hematology Am Soc Hematol Educ Program 2006.169-77. [PubMed]
- Schnittger S, Kinkelin U, Schoch C, et al. Screening for MLL tandem duplication in 387 unselected patients with AML identify a prognostically unfavorable subset of AML. Leukemia 2000;14:796-804. [PubMed]
- Becker H, Marcucci G, Maharry K, et al. Mutations of the Wilms tumor 1 gene (WT1) in older patients with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood 2010;116:788-92. [PubMed]
- Baldus CD, Tanner SM, Ruppert AS, et al. BAALC expression predicts clinical outcome of de novo acute myeloid leukemia patients with normal cytogenetics: a Cancer and Leukemia Group B Study. Blood 2003;102:1613-8. [PubMed]
- Takahashi S. Current findings for recurring mutations in acute myeloid leukemia. J Hematol Oncol 2011;4:36. [PubMed]
- Bienz M, Ludwig M, Leibundgut EO, et al. Risk assessment in patients with acute myeloid leukemia and a normal karyotype. Clin Cancer Res 2005;11:1416-24. [PubMed]
- Carow CE, Levenstein M, Kaufmann SH, et al. Expression of the hematopoietic growth factor receptor FLT3 (STK-1/Flk2) in human leukemias. Blood 1996;87:1089-96. [PubMed]
- Rosnet O, Bühring HJ, Marchetto S, et al. Human FLT3/FLK2 receptor tyrosine kinase is expressed at the surface of normal and malignant hematopoietic cells. Leukemia 1996;10:238-48. [PubMed]
- Weisberg E, Barrett R, Liu Q, et al. FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat 2009;12:81-9. [PubMed]
- Hannum C, Culpepper J, Campbell D, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature 1994;368:643-8. [PubMed]
- Lyman SD, Jacobsen SE. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood 1998;91:1101-34. [PubMed]
- Schlenk RF, Döhner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med 2008;358:1909-18. [PubMed]
- Zwaan CM, Meshinchi S, Radich JP, et al. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood 2003;102:2387-94. [PubMed]
- Kiyoi H, Ohno R, Ueda R, et al. Mechanism of constitutive activation of FLT3 with internal tandem duplication in the juxtamembrane domain. Oncogene 2002;21:2555-63. [PubMed]
- Kiyoi H, Towatari M, Yokota S, et al. Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia 1998;12:1333-7. [PubMed]
- Griffith J, Black J, Faerman C, et al. The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol Cell 2004;13:169-78. [PubMed]
- Zheng R, Bailey E, Nguyen B, et al. Further activation of FLT3 mutants by FLT3 ligand. Oncogene 2011;30:4004-14. [PubMed]
- Bacher U, Haferlach C, Kern W, et al. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters--an analysis of 3082 patients. Blood 2008;111:2527-37. [PubMed]
- Yamamoto Y, Kiyoi H, Nakano Y, et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood 2001;97:2434-9. [PubMed]
- Abu-Duhier FM, Goodeve AC, Wilson GA, et al. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol 2001;113:983-8. [PubMed]
- Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med 2009;361:1249-59. [PubMed]
- Fröhling S, Schlenk RF, Breitruck J, et al. Prognostic significance of activating FLT3 mutations in younger adults (16 to 60 years) with acute myeloid leukemia and normal cytogenetics: a study of the AML Study Group Ulm. Blood 2002;100:4372-80. [PubMed]
- Kottaridis PD, Gale RE, Frew ME, et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001;98:1752-9. [PubMed]
- Ravandi F, Kantarjian H, Faderl S, et al. Outcome of patients with FLT3-mutated acute myeloid leukemia in first relapse. Leuk Res 2010;34:752-6. [PubMed]
- Schnittger S, Schoch C, Dugas M, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 2002;100:59-66. [PubMed]
- Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002;99:4326-35. [PubMed]
- Stirewalt DL, Kopecky KJ, Meshinchi S, et al. Size of FLT3 internal tandem duplication has prognostic significance in patients with acute myeloid leukemia. Blood 2006;107:3724-6. [PubMed]
- Kayser S, Schlenk RF, Londono MC, et al. Insertion of FLT3 internal tandem duplication in the tyrosine kinase domain-1 is associated with resistance to chemotherapy and inferior outcome. Blood 2009;114:2386-92. [PubMed]
- Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer Res 2001;61:7233-9. [PubMed]
- Jin G, Matsushita H, Asai S, et al. FLT3-ITD induces ara-C resistance in myeloid leukemic cells through the repression of the ENT1 expression. Biochem Biophys Res Commun 2009;390:1001-6. [PubMed]
- Martinelli G. Effect of quizartinib (AC220) on response rates and long-term survival in elderly patients with FLT3-ITD positive or negative relapsed/refractory acute myeloid leukemia. Leukemia, Myelodysplasia, and Transplantation Track 2013 ASCO Annual Meeting.
- Levis MJ, Perl AE, Dombret H, et al. Final Results of a Phase 2 Open-Label, Monotherapy Efficacy and Safety Study of Quizartinib (AC220) in Patients with FLT3-ITD Positive or Negative Relapsed/Refractory Acute Myeloid Leukemia After Second-Line Chemotherapy or Hematopoietic Stem Cell Transplantation. ASH Annual Meeting and Exposition 2012;673.
- Perl AE, Dohner H, Rousselot PH, et al. Efficacy and safety of quizartinib (AC220) in patients age ≥70 years with FLT3-ITD positive or negative relapsed/refractory acute myeloid leukemia (AML). J Clin Oncol 2013;31:abstr 7023.
- Stone RM, Fischer T, Paquette R, et al. Phase IB study of the FLT3 kinase inhibitor midostaurin with chemotherapy in younger newly diagnosed adult patients with acute myeloid leukemia. Leukemia 2012;26:2061-8. [PubMed]
- Nazha A, Kantarjian HM, Borthakur G, et al. A phase I/II trial of combination of PKC412 and 5-azacytidine (AZA) for the treatment of patients with refractory or relapsed (R/R) acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). J Clin Oncol 2012;30:abstr 6589.
- Cooper BW, Kindwall-Keller TL, Lazarus HM, et al. Phase I study of midostaurin and azacitidine in relapsed and elderly AML. J Clin Oncol 2012;30:abstr 6589.
- Fiedler W, Kayser S, Kebenko M, et al. Sunitinib and Intensive Chemotherapy in Patients with Acute Myeloid Leukemia and Activating FLT3 Mutations: Results of the AMLSG 10-07 Study (ClinicalTrials.gov No. NCT00783653). ASH Annual Meeting and Exposition 2012;1483.
- Shah NP, Talpaz M, Deininger MW, et al. Ponatinib in patients with refractory acute myeloid leukaemia: findings from a phase 1 study. Br J Haematol 2013;162:548-52. [PubMed]
- Ravandi F, Alattar ML, Grunwald MR, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood 2013;121:4655-62. [PubMed]
- Metzelder S, Wang Y, Wollmer E, et al. Compassionate use of sorafenib in FLT3-ITD-positive acute myeloid leukemia: sustained regression before and after allogeneic stem cell transplantation. Blood 2009;113:6567-71. [PubMed]
- Jain N, Curran E, Iyengar NM, et al. Phase II study of the oral MEK inhibitor selumetinib (AZD6244) in advanced acute myeloid leukemia (AML). J Clin Oncol 2012;30:abstr 6582.
- Smith CC, Lasater E, Mccreery M, et al. Crenolanib (CP-868,596) Is a Potent and Selective Type I FLT3 Inhibitor That Retains Activity Against AC220 Resistance-Causing FLT3 Kinase Domain Mutants. ASH Annual Meeting and Exposition 2012;141.
- Gao C, Zhang W, Jacamo R, et al. Combination of Crenolanib with Sorafenib Produces Synergistic Pro-Apoptotic Effects in FLT3-ITD-Inhibitor-Resistant Acute Myelogenous Leukemias with FLT3 Mutations. ASH Annual Meeting and Exposition 2012;3591.
- Eriksson A, Hermanson M, Wickström M, et al. The novel tyrosine kinase inhibitor AKN-028 has significant antileukemic activity in cell lines and primary cultures of acute myeloid leukemia. Blood Cancer J 2012;2:e81. [PubMed]
- Wall HS, Nguyen BM, Li L, et al. TTT-3002 Is a Novel FLT3 Tyrosine Kinase Inhibitor That Has the Potential to Overcome Some of the Limitations of Current FLT3 Inhibitors in Treatment of Acute Myeloid Leukemia. ASH Annual Meeting and Exposition 2012;866.
- Li J, Bresnahan G, Gammon G, et al. Absorption, Metabolism, and Excretion of Quizartinib (AC220), a FLT3 Tyrosine Kinase Inhibitor for Treatment of Acute Myeloid Leukemia, in Healthy Male Volunteers. Blood (ASH Annual Meeting Abstracts) 2012;120:abstr 4327.
- Cortes JE, Perl AE, Dombret H, et al. Final Results of a Phase 2 Open-Label, Monotherapy Efficacy and Safety Study of Quizartinib (AC220) in Patients 60 Years of Age with FLT3 ITD Positive or Negative Relapsed/Refractory Acute Myeloid Leukemia. ASH Annual Meeting and Exposition 2012;48.
- Cortes JE, Perl AE, Dombret H, et al. Response rate and bridging to hematopoietic stem cell transplantation (HSCT) with quizartinib (AC220) in patients with FLT3-ITD positive or negative relapsed/refractory AML after second-line chemotherapy or previous bone marrow transplant. J Clin Oncol 2013;31:abstr 7012.
- Lamba G, Gupta R, Lee B, et al. Current management and prognostic features for gastrointestinal stromal tumor (GIST). Exp Hematol Oncol 2012;1:14. [PubMed]
- Gozgit JM, Wong MJ, Wardwell S, et al. Potent activity of ponatinib (AP24534) in models of FLT3-driven acute myeloid leukemia and other hematologic malignancies. Mol Cancer Ther 2011;10:1028-35. [PubMed]
- Wolleschak D, Schalk E, Krogel C, et al. Rapid induction of complete molecular remission by sequential therapy with LDAC and sorafenib in FLT3-ITD-positive patients unfit for intensive treatment: two cases and review of the literature. J Hematol Oncol 2013;6:39. [PubMed]
- Akinleye A, Furqan M, Mukhi N, et al. MEK and the inhibitors: from bench to bedside. J Hematol Oncol 2013;6:27. [PubMed]
- Grunwald MR, Levis MJ; Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, Baltimore, MD. Variations in FLT3 ligand levels during the course of AML treatment. J Clin Oncol 2013;31:abstr 7026.
Cite this article as: Pawar R, Bali OP, Malhotra BK, Lamba G. Recent advances and novel agents for FLT3 mutated acute myeloid leukemia. Stem Cell Investig 2014;1:7.


