Targeting Notch signalling pathway of cancer stem cells
Introduction
Cancer stem cells (CSCs) are the undifferentiated cell of tumor cell with infinite proliferation, self-renew, differentiation and growth. These stem cells play a vital role in growth and development of the cancerous tumors. Many studies have shown that the CSCs are the progenitor of the tumor and are the reason for recurrence of cancer. CSCs being chemo and radio resistance have shown metastases several years after curative treatment of the tumor which leads to recurrence (1). Current cancer treatment has minimum survival rate as these conventional cancer therapies target neoplastic cells that are largely fast-growing in abnormally, suggesting that CSCs may survive due to their high resistance to drugs and slower proliferation rate which leads to treatment failure (2,3). There are various molecular mechanisms i.e. signaling pathways that control and regulate the development of stem cells, the abnormal activity of these pathways regulates the growth, differentiation and development of these CSCs that include Notch which is vital to tumorigenicity of the CSCs (4,5). The blockage of development cellular pathway may be important as targeted therapy so as to obstruct the growth of the tumor cells. Herein, Notch signaling pathway is targeted which is juxtacrine signaling pathway by inhibitors that have undergone the clinical trials.
Role of CSCs in metastasis
CSCs are the cancer cells that possess characteristics associated with normal stem cells, specifically the ability to give rise to all cell types found in a particular cancer sample. CSCs also called as cancer initiating cells and tumor stem cells, they are the driving force of tumor development, initiation of invasion and metastasis as well as recurrence. CSCs differentiate and generate various tumor cells under the regulation of various signaling pathway that play a vital role in the development process (6,7).
CSCs might derive from a normal tissue stem cell that undergoes transformation as a result of oncogenic somatic mutations, under the influence of extrinsic micro environmental factors. Tumor cell progression towards metastasis is complex, multistage process, which classically acquire epithelial-mesenchymal transition (EMT) allowing them to disseminate from primary tumor and intravasate into the circulation as local invasion, then they have to survive in the circulation later they extravasate and colonize into foreign tissues, these cells adapt to the microenvironment and they form macro metastasis, i.e., CSC may arise from transformed epithelial cells through an EMT process to acquire migratory and metastatic properties (7).
In various experiments conducted, CSCs seem to be more resistant to chemotherapy and radiotherapy than ‘differentiated’ tumor cells. Indeed, CSCs residing in micro environmental niches can escape from the effects of conventional cytotoxic treatments. Expansion of the remaining highly tumorigenic CSCs can resume after treatment, which is clinically relapsed or reoccurred. The tumorigenic capacity was studied by injecting the CSC in limiting dilutions into immunodeficient mice. On the basis of these theories and observations, numerous researchers hypothesize that treatments targeting the CSC population could be more effective than existing therapies, and could dramatically transform treatment outcomes in oncology (5).
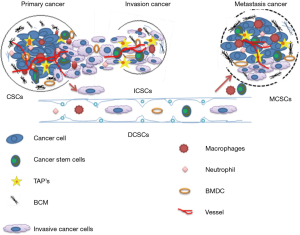
Figure 1 explains the evolving concept of MCSCs (metastatic CSCs), ICSCs (invasive CSCs) invading into the ECMs (extracellular matrix) and penetrating into blood vessels, DCSCs (disseminating CSC) surviving and disseminating in the circulation, MCSCs undergoing extravasations and forming colonization at the distant organs (7).
Various reports demonstrate that there are various CSC markers that play a crucial role in metastases and enhance their capabilities like CD44 and chemokine receptor CXCR4 expression by breast cancer tumor cells and CD44 expression in pancreatic tumor cells (8-11).
Signaling pathway such as Wnt, Notch and Hedgehog (Hh) are crucial for the regulation of EMT or metastasis and self-renew of CSCs in several cancers. This strategy of signaling pathway for the growth of tumor cells can be inhibited by inhibitors that block the signals for the growth and proliferation of the CSCs, which may act as a blockade for the metastasis, and recurrence that is effective therapy (7).
Notch signalling pathway
CSCs could self-renew and generate more differentiated cancer progenitor on replication, cancer progenitor cells have the capacity to dedifferentiate and acquire a stem-like phenotype by a series of mechanisms, such as the microenvironment, signaling pathways, molecular circuitries and epigenetic modifications.
Notch signaling pathway is highly conserved molecular cell signaling pathway that regulates a vital role in proliferation, stem cell maintenance, cell fate specification, differentiation, and homeostasis of multicellular organism and implicates angiogenesis. They play a key role in embryonic vasculature development (12). Notch signaling is one of the most activated pathways in cancer cells, which have been experimentally demonstrated that they contribute to the cancer metastasis. Notch pathway plays a critical role in the linkages between angiogenesis and CSCs self-renewal and is thus receiving increased attention as a target to eliminate CSCs. The inhibition of this pathway may contribute to the therapeutic strategy to cure cancer by completely eradicating the CSCs (8) (Figure 2).
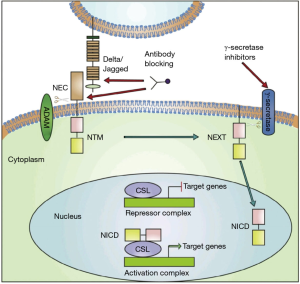
Targeting Notch signaling pathway
Features of Notch signaling pathway has few unique relevance that has to be taken into consideration for targeting the pathway, Notch-ligand interaction not being enzymatic the ‘signal intensity’ can be modulated precisely by cellular regulatory mechanisms. Complete shutdown of the pathway is not necessary for the therapeutic purpose (13,14) (Table 1).

Full table
There are various data that suggests that the Notch pathway plays a fundamental role in the development and maintenance of the hematopoietic system by playing central role in cell fate in which they support the growth, proliferation and differentiation of the stem cells. There is also evidence that the Notch pathway affects cell fate choices at various stages of hematopoietic cell development, including the decisions of Hematopoietic stem cells of the cancer tumor to self-renew or differentiate and of common lymphoid precursors to undergo T- or B-cell differentiation (17,24) (Figure 3).
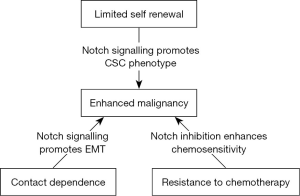
Notch signaling could be targeted by inhibiting the signaling pathway by two major classes of notch inhibitors that primarily focuses on the clinical development of promising agents that either obstruct Notch receptor cleavages such as γ-secretase inhibitors (GSIs) or interfere with the Notch ligand-receptor interaction by monoclonal antibodies (mAbs). Combining notch inhibitors with current cancer therapies can be effective method for treatment strategies that can give most promising results.
Targeting Notch signaling has the potential to affect multiple cell types within a tumor like CSCs to immune cells, the successful development of agents targeting the Notch pathway will require an understanding of the role of Notch signaling in specific cancers and ideally, the development and use of mechanism-based combination regimens. The main issues in the development of agents targeting Notch signaling in oncology include: choice of the most appropriate inhibitor for each patient, identification of pharmacodynamic biomarkers, selection of mechanism-based combination regimen and patient stratification according to recognized efficacy biomarkers. Antitumor activity has seen when both GSI and mAb are administered as a single agent in early stage of development of cancers like thyroid cancer, lung cancer, intracranial tumors, sarcoma, colorectal cancer, etc. (14).
In a study they confirmed that Notch1 inhibition delays tumorigenesis and effectively reduces CSC self-renewal of nude mice Head and Neck squamous cell carcinoma xenograft. The synergistic effect of Notch inhibitor and chemotherapy drug attenuated chemotherapy-enriched CSC population in vitro and in vivo, which provides the possibility to effectively eliminate head and neck CSCs.
GSI
γ-secretase is a large protease complex and is composed of catalytic and accessory subunits. The activation of Notch signaling pathway mainly depends on the γ-secretase enzyme activity that helps in the proteolytic cleavage of the receptors that release the active intracellular fragment which is one of the most crucial steps. Thus, GSIs is a promising target for Notch inhibition. GSI were the first class of inhibitors that reached clinical development in oncology. There are more than 100 GSIs synthesized and they can be divided into three classes: peptide isosteres, azepines and sulfonamides. Azepines and sulfonamides are most popular and widely used (14).
In various preclinical studies these agents have shown strong anti-neoplastic activities, anti-angiogenesis, anti-CSC effects, anti-tumor growth and apoptosis especially in combination with chemotherapeutic or targeted drugs (25).
Studies conducted showed that using multiple myeloma cell lines and primary multiple myeloma cells isolated from the bone marrow of patient found a strong synergistic effect of bortezomib in combination with one of the GSIs, which can be successfully used as anti-multiple myeloma drug which may enhance the mechanism inhibition of proteasome activity (18). This proves that GSIs can effectively repress the CSC that inhibit the recurrence of cancer by deregulating the angiogenesis and this triggers apoptosis of tumor cells by inhibiting proteasome activity and enhancing endoplasmic reticulum stress. This can be used as a promising therapy for the cure of multiple myeloma.
The well know toxicities or side effects of GSIs is associated with gastrointestinal tract, that cause the increase in goblet containing mucin in large and small intestine that leads to severe adverse diarrhea. So, they are dose limiting and require moderate, intermittent administration.
Studies demonstrated that Notch signaling inhibition using a GSI (GSI-IX, DAPT) decreased tumor burden in the mouse model after prophylactic treatment. In addition, flow cytometry analysis indicated that Notch signaling inhibition reduced the sub-population of myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and regulatory T cells (Tregs) in the circulation and in the tumor (20).
Oral GSI inhibits Notch pathway activity and expression of stem cell markers; this was proved by measuring the expression of established pathway targets in tumor-derived mRNA in three xenograft cohorts (21).
RO4929097 (RG-4733) is a novel gamma secretase inhibitor being studied as an anti-cancer drug, which is a key component of the enzymatic complex that cleaves and activates Notch (22). The drug was initially developed by Roche for the treatment of Alzheimer’s disease, but current research focuses on cancer. Over 35 phases I and II clinical trials have been performed but no phase III trials have yet commenced (23). Phase II studies have investigated the use of RO4929097 in ovarian cancer which when used as immunotherapy inhibits Notch pathway (26), renal cell carcinoma patients with solid tumor (27,28) metastatic pancreatic cancer (29), advanced brain tumors (30), and relapsed non-small cell lung cancer. The effects of RO4929097 on the oncogenic and stem cell properties of a panel of melanoma cell lines were tested both in vitro and in vivo, using xenograft models. Increased gene expression of Notch signaling components correlated with shorter post recurrence survival in metastatic melanoma cases (9). The most common severe adverse events were nausea, fatigue and anemia in patients with advanced cancer (31). Various research studies show that RO4929097 in phase 1 study can be safely used in combination with other agents like temsirolimus in patients with advanced solid tumors (32), cediranib which is a vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitor, showed antitumor activity with solid tumors (28), gemcitabine showed clinical antitumor activity and >4 months stable disease in pancreas, tracheal and primary breast cancer (29). Phase I study of RO4929097 in combination with bevacizumab in patients with recurrent malignant glioma showed anti-angiogenic effect with well tolerated synergic activity (30) (Table 2).

Full table
PF-03084014 is a small molecule reversible, non-competitive and selective GSI that inhibits growth of several T-acute lymphoblastic leukemia (T-ALL) cell lines by inducing cell cycle arrest at the G0–G1 phase and induction of apoptosis in preclinical model (33).
An in vivo study shows that PF-03084014 when orally administrated in a single dose of 200 mg/kg causes maximal Notch intracellular domain (NICD) inhibition for ~80% in xenograft HPB-ALL tumors. PF-03084014 shows robust antitumor activity in this mode with a maximal tumor growth inhibition, cell growth inhibition with cell cycle arrest, apoptosis and they also caused significant reduction of NICD levels and dose dependent tumor growth inhibition in HPB-ALL tumors. The intermittent dosing reduced gastrointestinal toxicity (33).
Phase I study of the GSI PF-03084014 in combination with docetaxel in patients with advanced triple negative breast cancer, the maximum tolerated dose (MTD) was estimated to be 100 mg twice daily/docetaxel 75 mg/m2. At this dose level, combination treatment was generally well tolerated. The most common, treatment-related adverse events in patients reported were neutropenia, fatigue, nausea, leukopenia, diarrhea, alopecia, anaemia and vomiting. No effect was observed on the pharmacokinetics of docetaxel when administered in combination with PF-03084014. They exhibited anti-tumor activity of this combination in xenograft models (34).
PF-03084014 (120 mg/kg) induces apoptosis, anti-angiogenesis, anti-proliferation, reduces tumor cell self-renewal ability, impairs tumor vasculature, and decreases metastasis activity in breast cancer HCC1599 tumor-bearing mice. PF-03084014 treatment displays significant antitumor activity in various types of the breast xenograft models with TGI (tumor growth inhibition) value of at least 50% in phase I clinical trials (35).
When PF-03084014 was combined with gemcitabine it resulted in tumor regression in 3 of 4 pancreatic cancer xenograft models via targeting malignant CSCs. The combined therapy showed enhanced efficacy in the inhibition of tumor cell proliferation and angiogenesis, attenuating (19) growth of primary tumor and controlling metastatic dissemination in a highly aggressive orthotopic model (36). The toxicities of PF-03084014 were generally mild or moderate diarrhea, nausea, fatigue, hypophosphatemia, and vomiting, rash, decreased appetite and mainly related to gastrointestinal problems (19,33-35,37).
MK-0752, is a potent non-competitive oral inhibitor of γ-secretase which prevents cleavage of gamma-secretase substrates with a 50% inhibitory concentration (IC50) (35) (Table 3).
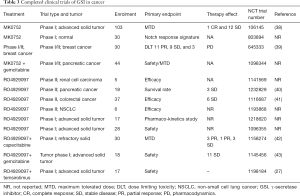
Full table
MK-0752 is well-tolerated and exhibits target inhibition in children with recurrent central nervous system (CNS) malignancies supporting a rational application (44). MRK003 is structurally similar to MK0572 and is used in preclinical in vivo studies of both solid tumors and leukemia as it has superior pharmacokinetic properties in rodents (21).
Pre-treatment of pancreatic ductal adenocarcinoma (PDAC) cells with MRK-003 in cell culture significantly inhibits the subsequent engraftment in immunocompromised mice. MRK-003 monotherapy significantly blocked tumor growth in 5 of 9 (56%) PDAC xenografts. A combination of MRK-003 and gemcitabine showed enhanced antitumor effects compared to gemcitabine in 4 of 9 (44%) PDAC xenografts reduced tumor cell proliferation and induced both apoptosis and intra-tumoral necrosis (45). The patients with advanced solid tumors MK0572 GSI were administered weekly and this dosing was well tolerated and resulted in clinical benefit (38).
Weekly oral delivery of MRK003 which is GSI results in significant in vivo inhibition of Notch pathway activity, tumor growth, stem cell marker expression, and clonogenicity, providing preclinical support for the use of such compounds in patients with malignant brain tumors. MRK003 slows growth of glioblastoma xenografts and prolongs survival (21).
Clinical trial treatment with GSI MK-0752 enhanced the efficacy of docetaxel in preclinical studies. The patients with advanced breast cancer were treated with escalating doses of MK-0752 plus docetaxel. Clinically, meaningful doses of both drugs were possible with manageable toxicity and preliminary evidence of efficacy which showed reduced CSC subpopulation in vitro and in human tissue. The most common side effects include diarrhea, nausea, vomiting, and fatigue. MK-0752 toxicity was schedule dependent. Weekly dosing was generally well tolerated and resulted in strong modulation of a Notch gene signature (37). Clinical benefit was observed, and rational combination trials are currently ongoing to maximize clinical benefit with this novel agent.
mAb inhibitors
These are mAbs tested in clinical, which recognize specific ligand (DLL-4) or receptors (Notch1–3) and they either prevent ligand/ receptor interaction or the conformational change within the extracellular domain which is required to expose the TACE cleavage site (46). Delta like ligand 4 (DLL-4) is an important component of NOTCH pathway that controls the proper growth, stem cell renewal and development. Any deletion in the gene that codes DLL-4 shows a lethal effect on the vasculature, i.e., abnormalities and over expression of DLL-4 is found in tumor vasculature and in tumor cells to activate Notch signaling for their further active growth (12,32,47). Various xenograft models showed that blockade of specific DLL-4 in combination with ionizing radiation retards the tumor growth activity of cancer cell lines by promoting nonproductive tumor angiogenesis and extensive tumor necrosis thereby reducing tumor-initiating cell frequency and delayed tumor recurrence (32,48,49).Data indicated that that DLL-4-mediated Notch signaling is crucial during active vascularisation, but less important for normal vessel maintenance and the antibodies against DLL-4 and antibodies against VEGF had distinct effects on tumour vasculature (32).Thus, it can be concluded that inhibiting these components one can successfully provide a promising therapeutic strategy to cure the cancer, targeting these components in CSCs. They have developed various mAbs such as trastuzumab, pertuzumab, rituximab, bevacizumab and others which have gained clinical acceptances part of combination regimens that can be targeted to the DLL-4 of the CSCs. Some have been proved to be effective and even with these agents resistance is observed (15,16,50).
Experimental evidence shows that notch inhibition by mAb against Notch1 or 2 alleviates the toxicities while inhibition of Notch1 and 2 leads to severe gastrointestinal toxicities (10). Two classes of blocking anti-Notch antibodies have been developed. One is directed to the extracellular negative regulator region (NRR) of notch blocking, the conformational change that allows the ADAM protease cleavage and the other is the ligand-competitors directed against the EGF-repeat region of notch receptors, blocking the ligand binding domain (LBD). Both NRR and LBD notch antibodies induce a strong and specific down regulation of notch 1 signaling. Studies using murine tumor models showed anti-angiogenic effect, reduce the blood circulation to the tumor cells and finally lead to retardation of tumor growth (32,43,51).
Development of Humanized mAbs OMP-59R5 (tarextumab) which alone or together with chemotherapeutic agents can inhibit Notch2 and Notch3 function. The antitumor effect of OMP-59R5 was observed on xenograft tumors representing different types of epithelial cancers like breast, small-cell lung, ovarian, and pancreatic was associated with down-regulation of Notch target genes in tumor cells and with suppression of Notch3, HeyL, and Rgs5, expression in tumor stroma and vasculature (52-54). OMP-59R5 inhibits xenograft tumor growth through reducing tumor cell proliferation and promotes differentiation this was accessed using passaged patients derived xenografts (PDX) models. They alone showed 40% of reduced proliferation. OMP-59R5 reduces CSC frequency and delays tumor recurrence after termination of chemotherapy. The antitumor effect of anti-Notch2/3 in combination with gemcitabine plus nab-paclitaxel was greater than the combination effect with gemcitabine alone. The MTDs of OMP-59R5 were observed to be 2.5 mg/kg weekly and 7.5 mg/kg every three weeks 10 (55).
The mAbs REGN421 and SAR153192 (also called enoticumab) had a reasonable safety profile and demonstrated preliminary efficacy signals. REGN421 is a fully humanized IgG1mAb that binds to human DLL-4 and disrupts Notch-mediated signaling (56). The most-common adverse effects were fatigue, headache, hypertension and nausea reversible severe adverse events included increased levels of the cardiac proteins natriuretic peptides B and troponin I and right and left ventricular dysfunctions. Partial responses and stable response were observed among the patients with ovarian cancer and other solid tumors who were treated with enoticumab (43,56,57) (Tables 4,5).

Full table
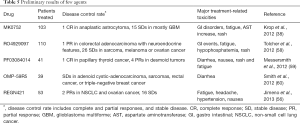
Full table
Discussion
Notch signaling pathway is a conserved cell fate determining factor in embryo development. Notch pathway plays an important role in linkage between angiogenesis and CSCs self-renewal. CSCs are population of cells that have stem cell properties and invasion capabilities that contribute to cancer metastasis. CSC escape from immune surveillance and they survive from radiation and chemotherapy later they result in reconstruction of cancer tissue. This concept of targeting notch signaling pathway as a therapeutic strategy for treating cancer has attracted increasing interest. Cell signaling pathway such as notch, they critically regulate the self-renewal and survival of CSC. Notch pathway is deregulated in CSC using inhibitors that inhibit the signaling for development of further tumor cells. CSC eradication is an important goal in curing cancer as CSC result in reconstruction of cancer tissue even after successful conventional treatment, thus Notch Pathway is attractive target for treatment as they kill differentiated cancer cells but could also kill CSCs.
Since the activity of Notch is activated via gamma secretase, it is targeted and inhibited using GSI which effectively repress CSC and inhibit recurrence of cancer by retardation of angiogenesis and triggering apoptosis of tumor cells.
Another way to target Notch signaling pathway is by blocking DLL-4 that plays an important role in growth, renewal and development. Blockade of this DLL-4 using mAb in combination with either ionizing radiation or chemotherapy drugs retards tumor growth of CSC promoting tumor necrosis, reducing tumor initiating cell frequency, delayed tumor recurrence and nonfunctional tumor angiogenesis.
GSI and mAb cause adverse severe side effects. So, they are dose limiting and require moderate, intermittent administration.
In conclusion, Notch signaling inhibition of CSCs in tumor have opened a broad view for designing novel diagnostic and therapeutic strategies in order to detect and specifically target the cancer cells pathway that might bring future direction for the recurrence and cure of cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Malik B, Nie D. Cancer stem cells and resistance to chemo and radio therapy. Front Biosci (Elite Ed) 2012;4:2142-9. [Crossref] [PubMed]
- Holohan C, Van SS, Longley DB, et al. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer 2013;13:714-26. [Crossref] [PubMed]
- Lage H.. An overview of cancer multidrug resistance: A still unsolved problem. Cell Mol Life Sci 2008;65:3145-67. [Crossref] [PubMed]
- Hu YY, Zheng MH, Zhang R, et al. Notch signaling pathway and cancer metastasis. Adv Exp Med Biol 2012;727:186-98. [Crossref] [PubMed]
- Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol 2015;12:445-64. [Crossref] [PubMed]
- Dragu DL, Laura GN, Coralia B, et al. Therapies targeting cancer stem cells: Current trends and future challenges. World J Stem Cells 2015;7:1185-201. [PubMed]
- Liao WT, Ye Y, Deng Y, et al. Metastatic cancer stem cells: from the concept to therapeutics. Am J Stem Cells 2014;3:46-62. [PubMed]
- Al-Hajj M, Wicha SM, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci 2003;100:3983-8. [Crossref] [PubMed]
- Taichman RS, Cooper C, Keller ET, et al. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res 2002;62:1832-7. [PubMed]
- Müller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001;410:50-6. [Crossref] [PubMed]
- Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1:313-23. [Crossref] [PubMed]
- Patel NS, Li JL, Generaloi D, et al. Up-regulation of Delta-like 4 Ligand in Human Tumor Vasculature and the Role of Basal Expression in Endothelial Cell Function. Cancer Res 2005;65:8690-7. [Crossref] [PubMed]
- Miele L, Golde T, Osborne B. Notch signaling in cancer. Curr Mol Med 2006;6:905-18. [Crossref] [PubMed]
- Rizzo P, Osipo C, Foreman K, et al. Rational targeting of Notch signaling in cancer. Oncogene 2008;27:5124-31. [Crossref] [PubMed]
- Capaccione KM, Pine SR. The Notch signaling pathway as a mediator of tumor survival. Carcinogenesis 2013;34:1420-30. [Crossref] [PubMed]
- Milas L, Hittelman WN. Cancer stem cells and tumor response to therapy: current problems and future prospects. Semin Radiat Oncol 2009;19:96-105. [Crossref] [PubMed]
- Varnum-Finney B, Xu L, Brashem-Stein C, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med 2000;6:1278-81. [Crossref] [PubMed]
- Chen F, Pisklakov A, Li M, et al. Gamma-secretase inhibitor enhances the cytotoxic effect of bortezomib in multiple myeloma. Cell Oncol (Dordr) 2011;34:545-51. [Crossref] [PubMed]
- Zou J, Li P, Lu F, et al. Notch1 is required for hypoxia-induced proliferation, invasion and chemoresistance of T-cell acute lymphoblastic leukemia cells. J Hematol Oncol 2013;6:3. [Crossref] [PubMed]
- Mao L, Zhao ZL, Yu GT, et al. γ-Secretase inhibitor reduces immunosuppressive cells and enhances tumour immunity in head and neck squamous cell carcinoma. Int J Cancer 2018;142:999-1009. [Crossref] [PubMed]
- Chu Q, Orr AB, Semenkow S, et al. Prolonged inhibition of glioblastoma xenograft initiation and clonogenic growth following in vivo Notch blockade. Clin Cancer Res 2013;19:3224-33. [Crossref] [PubMed]
- Huynh C, Poliseno L, Segura FM, et al. The novel gamma secretase inhibitor RO4929097 reduces the tumor initiating potential of melanoma. PLoS One 2011;6:e25264. [Crossref] [PubMed]
- Available online: https://clinicaltrials.gov
- Ohishi K, Varnum-Finney B, Bernstein ID. The notch pathway: modulation of cell fate decisions in hematopoiesis. Int J Hematol 2002;75:449-59. [Crossref] [PubMed]
- Curry CL, Reed LL, Golde TE, et al. Gamma secretase inhibitor blocks Notch activation and induces apoptosis in Kaposi's sarcoma tumor cells. Oncogene 2005;24:6333-44. [Crossref] [PubMed]
- Messersmith WA, Shapiro GI, Clearly JM, et al. A phase I, dose-finding study in patients with advanced solid malignancies of the oral gamma-secretase inhibitor. Clin Cancer Res 2015;21:60-7. [Crossref] [PubMed]
- Diaz-Padilla I, Hirte H, Oza AM, et al. A phase Ib combination study of RO4929097, a gamma-secretase inhibitor, and temsirolimus in patients with advanced solid tumors. Invest New Drugs 2013;31:1182-91. [Crossref] [PubMed]
- Sahebjam S, Bedard LP, Castonguay V, et al. A phase I study of the combination of ro4929097 and cediranib in patients with advanced solid tumours. Br J Cancer 2013;109:943-9. [Crossref] [PubMed]
- Richter S, Bedard PL, Chen X, et al. A phase I study of the oral gamma secretase inhibitor R04929097 in combination with gemcitabine in patients with advanced solid tumors. Invest New Drugs 2014;32:243-9. [Crossref] [PubMed]
- Pan E, Supko JG, Kaley TJ, et al. Phase I study of RO4929097 with bevacizumab in patients with recurrent malignant glioma. J. Neurooncol 2016;130:571-9. [Crossref] [PubMed]
- Lee SM, Moon J, Redman BG, et al. Phase 2 study of RO4929097, a gamma-secretase inhibitor, in metastatic melanoma. Cancer 2015;121:432-40. [Crossref] [PubMed]
- Ridgway J, Zhang G, Wu Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulatin angiogenesis. Nature 2006;444:1083-7. [Crossref] [PubMed]
- Wei P, Walls M, Qiu M, et al. Evaluation of selective gamma-secretase inhibitor PF-03084014 for its antitumor efficacy and gastrointestinal safety to guide optimal clinical trial design. Mol Cancer Ther 2010;9:1618-28. [Crossref] [PubMed]
- Locatelli MA, Aftimos P, Dees CE, et al. Phase I study of the gamma secretase inhibitor PF-03084014 in combination with docetaxel in patients with advanced triple-negative breast cancer. Oncotarget 2017;8:2320-8. [Crossref] [PubMed]
- Zhang CC, Pavlick A, Zhang Q, et al. Biomarker and pharmacologic evaluation of gamma-secretase inhibitor PF-3084014 in breast cancer models. Clin Cancer Res 2012;18:5008-19. [Crossref] [PubMed]
- Yabuuchi S, Pai GS, Campbell RN, et al. Notch signaling pathway targeted therapy represses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett 2013;335:41-51. [Crossref] [PubMed]
- LoRusso P, Demuth T, Heath E, et al. Phase I study of the gamma secretase inhibitor MK-0752 in patients with metastatic breast and other advanced solid tumors. 100th AACR Annual Meeting, Denver, CO. Apr 18-22, 2009.
- Krop I, Demuth T, Guthrie T, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol 2012;30:2307-13. [Crossref] [PubMed]
- Schott AF, Landis MD, Dontu G, et al. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin Cancer Res 2013;19:1512-24. [Crossref] [PubMed]
- De Jesus-Acosta A, Laheru D, Maitra A, et al. A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest New Drugs 2014;32:739-45. [Crossref] [PubMed]
- Strosberg JR, Yeatman T, Weber J, et al. A phase II study of RO4929097 in metastatic colorectal cancer. Eur J Cancer 2012;48:997-1003. [Crossref] [PubMed]
- Deenen MJ, Klümpen HJ, Richel DJ, et al. Phase I and pharmacokinetic study of capecitabine and the oral mTOR inhibitor everolimus in patients with advanced solid malignancies. Invest New Drugs 2012;30:1557-65. [Crossref] [PubMed]
- Wu Y, Cain-Hom C, Choy L, et al. Therapeutic antibody targeting of individual Notch receptors. Nature 2010;464:1052-7. [Crossref] [PubMed]
- Hoffman LM, Vekemans J, Richie TL, et al. Phase I Trial of Weekly MK-0752 in Children with Refractory Central Nervous System Malignancies: A Pediatric Brain Tumor Consortium Study. Childs Nerv Syst 2015;31:1283-9. [Crossref] [PubMed]
- Mizuma M, Rasheed AZ, Yabuuchi S, et al. The gamma secretase inhibitor MRK-003 attenuates pancreatic cancer growth in preclinical models. Mol Cancer Ther 2012;11:1999-2009. [Crossref] [PubMed]
- Brennan K, Clarke BR. Combining Notch inhibition with current therapies for breast cancer treatment. Ther Adv Med Oncol 2013;5:17-24. [Crossref] [PubMed]
- Duarte A, Hirashima M, Benedito R, et al. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev 2004;18:2474-8. [Crossref] [PubMed]
- Noguera-Troise I, Daly C, Papadopoulos NJ, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 2006;444:1032-7. [Crossref] [PubMed]
- Liu SK, Bham SA, Fokas E, et al. Delta-like ligand 4-notch blockade and tumor radiation response. J Natl Cancer Inst 2011;103:1778-98. [Crossref] [PubMed]
- Purow B.. Notch inhibition as a promising new approach to cancer therapy. Adv Exp Med Biol 2012;727:305-19. [Crossref] [PubMed]
- Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by Notch. Dev Cell 2009;16:196-208. [Crossref] [PubMed]
- Lin L, Mernaugh R, Yi F, et al. Targeting specific regions of the Notch3 ligand-binding domain induces apoptosis and inhibits tumor growth in lung cancer. Cancer research 2010;70:632-8. [Crossref] [PubMed]
- Park JT, Li M, Nakayama K, et al. Notch3 gene amplification in ovarian cance. Cancer Research 2006;66:6312-8. [Crossref] [PubMed]
- Mann DC, Bastianpillai C, Neal PC, et al. Expression of nuclear Notch3 in pancreatic adenocarcinomas is associated with adverse clinical features, and correlates with the expression of STAT3 and phosphorylated Akt. J SurgOnco 2012;97:63-8.
- Yen WC, Fischer MM, Axelrod F, et al. Targeting Notch signaling with a Notch2/Notch3 antagonist(tarextumab) inhibits tumor growth and decreases tumor initiating cell frequency. Clin Cancer Res 2015;21:2084-95. [Crossref] [PubMed]
- Jimeno A, LoRusso P, Strother MR, et al. Phase I study of REGN421 (R)/SAR153192, a fully-human delta-like ligand 4 (Dll4) monoclonal antibody (mAb), in patients with advanced solid tumors. J Clin Oncol 2013;31:2502.
- Yan M.. Therapeutic promise and challenges of targeting DLL4/NOTCH1. Vasc Cell 2011;3:17. [Crossref] [PubMed]
- Tolcher AW, Messersmith WA, Mikulski SM, et al. Phase I study of RO4929097, a gamma secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol 2012;30:2348-53. [Crossref] [PubMed]
- Messersmith W, LoRusso P, Cleary J, et al. A first-in-patient Phase I study of the novel gamma secretase inhibitor PF-03084014 in patients with advanced solid tumor malignancies. Eur J Cancer 2012;48:180. [Crossref]
- Smith DC, Chugh R, Patnaik A, et al. A first-in-human phase I study to evaluate the fully human monoclonal antibody OMP-59R5 (anti-Notch2/3) administered intravenously to patients with advanced solid tumors. Euro J Cancer 2012;48:11-2. [Crossref]
Cite this article as: Venkatesh V, Nataraj R, Thangaraj GS, Karthikeyan M, Gnanasekaran A, Kaginelli SB, Kuppanna G, Kallappa CG, Basalingappa KM. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig 2018;5:5.