Alopecia and platelet-derived therapies
Introduction
Hair loss disorders, such as androgenetic alopecia (AGA), display relatively high rates of occurrence in both men and women. Fifty percent of white men will experience some degree of AGA, also known as male-pattern baldness or MPHL, by age 50 (1), and nearly 50% of women will experience AGA (female-pattern hair loss, FPHL) over the course of her lifetime (2). Given the prevalence of hair loss in these populations, targeted therapies that reduce the appearance of thinning by delaying, arresting, or reversing the underlying pathology are highly desirable. However, the current selection of pharmacotherapies with US Food and Drug Administration (FDA) approval are limited to oral finasteride, a selective 5-α-reductase inhibitor, and 2% or 5% topical minoxidil solutions or foams (3,4), neither of which enable complete hair regrowth, even with persistent use. Moreover, the patient will inevitably face relapse in hair loss if and when the treatment is halted, and undesirable side effects may result from these medications, some of which, such as finasteride-induced impotence, may persist for many years (5).
Alternative cell-based therapies for the treatment of AGA have emerged, of which, platelet rich plasma (PRP) injections appear particularly effective. Although the precise degree to which PRP, improve hair density is difficult to extract from individual studies given the relatively small sample sizes and lack of standardization in follow-up periods, test factors measured, and instruments used to quantify hair count and/or quality, a recent meta-analysis identified PRP as a promising treatment for AGA (6). In practice, autologous PRP (A–PRP) may be applied directly or activated (AA–PRP) prior to delivery. When A–PRP has been injected in its native stage, hair density improvements have ranged from 13% to 31% over baseline measurements 3 months following the initial treatment (7,8). Treatment with AA–PRP injections has shown similar hair density improvements 12 to 14 weeks after therapy was begun, with hair densities ranging from 18% to 29% above baseline (9-11). However, hair density measurements made 6 months after the start of AA–PRP treatment have shown up to a 56% improvement over baseline values, although the degree of improvement seems to be influenced by the system used to generate the PRP from whole blood (7).
Since PRP therapies are still in their infancy, and have not been shown to arrest or reverse the hair loss process, many AGA patients turn to surgical intervention to conceal ongoing thinning (12). Within the hair restoration arena, the minimal depth follicular unit isolation technique, particularly when conducted with sharp punches, has proven to be cosmetically effective and affords markedly low transection rates in skilled hands (13). The “minimal depth” ranges from 1.8 to 3.0 mm below the scalp surface, which places the extraction punch in the deeper dermis or superficial adipose tissue at full stop. Since the average hair follicle extends 4.2 mm (range, 4–5.1 mm) into the scalp (14), the minimal depth extraction approach allows valuable cell populations to remain intact following follicular extraction, namely, epidermal stem cells distal to the sebaceous gland in the bulge region and mesenchymal stem cells in the dermal papilla (15).
In addition to the minimally invasive surgical procedure that conserves vital stem cell populations within the donor region of the scalp, drug therapies that promote wound healing and accelerate regeneration of transplanted hair follicles may also be applied. Products such as hyaluronic acid, extracellular matrix proteins, or PRP may be used either singularly or in an assortment of combinations (16). Uebel et al., for example, have immersed extracted grafts in A–PRP then activated the PRP immediately prior to graft implantation (17). In this work, grafts implanted with AA–PRP displayed significantly higher rates of survival over controls. To date, A–PRP activation, in the context of hair restoration surgeries, has been accomplished via thrombin, calcium chloride, or calcium gluconate solutions as a means to deliver GFs that are either secreted by the platelets or presented on the activated platelet surface (18). However, since each of these products constitute a unique drug therapy with accompanying unwanted or potentially hazardous side effects, an alternative process by which platelet contents may be isolated is warranted for efficacious clinical use. Platelet lysis and accumulation of the released lysate (PL) is one solution, which is accomplished herein via sonication. In this work, the survival rates of hair follicles treated with PL, AA–PRP, or normal saline following transplantation are compared, and the relative growth factor (GF) concentrations for PL and AA–PRP are evaluated.
Methods
Patient population
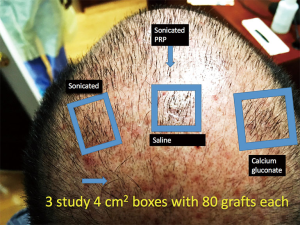
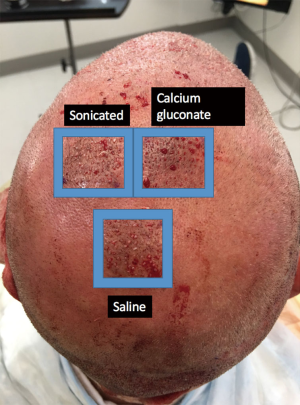
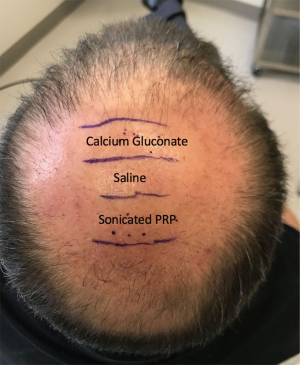
This study enrolled 3 male patients aged 27–55 years who displayed MPHL in Stage 6 as determined by the Norwood-Hamilton classification scale. For each patient, three sets of 2 cm × 2 cm (or 4 cm2 area) boxes were marked in three separate regions of the transplant zone. Follicular unit grafts in conjunction with 1.0 mL PL, 1.0 mL calcium gluconate-activated PRP (AA–PRP), or 1.0 mL normal saline were then placed in each box. The locations of the respective treatment, positive control, and negative control boxes for each patient are outlined in Figures 1–3. Note, however, that the number of hairs within each follicular unit were controlled only in subject C. Only the absolute number of grafts, not the absolute number of hairs was held constant among the three regions in subjects A and B. The frontal scalp was treated with 0.1 mL cm−2 PL in all patients. A second group of 5 subjects (3 male and 2 female aged 20–60 years) with no apparent hair loss was selected solely for analyzing GF concentrations in PL and AA–PRP.
A–PRP preparation, activation, and lysis
A–PRP was prepared using one of two systems. In the first, Regen Blood Cell Therapy (BCT) tubes were used to prepare A–PRP (15 and 5 mL per BCT tube) from whole blood (24 mL) taken from a peripheral vein using sodium citrate as an anticoagulant. The top 2 mL of A–PRP from each tube was then discarded, giving 9 mL of A–PRP with a 5-fold increase in platelet concentration over whole blood. The Regen system A–PRP was activated through the addition of 10% (v/v) calcium gluconate, and the resulting AA–PRP was immediately injected at a depth of 3 mm into the appropriate treatment zone through a 1 mL Luer lock syringe equipped with a 25-gauge needle.
The Arthrex Angel system was also used to prepare A–PRP (3 mL) from 120 mL of whole blood with the instrument hematocrit level set to 3%. The A-PRP was then combined with 5 mL of platelet poor plasma to produce 8 mL of A–PRP with a 5-fold increase in platelet concentration over whole blood. A–PRP collected with the Arthrex Angel system was subjected to 30 s on/30 s off sonication cycles using a Bioruptor Plus sonication device (Diagenode) equipped with six falcon 15 mL tube holders, each filled with 1.33 mL of A–PRP and maintained at 4 °C over the entire sonication period by an accompanying water cooler (115 V minichiller, Diagenode). Total sonication time was set to 30 min (i.e., 60 on/off cycles). Upon completion of the final cycle, the lysed platelet samples were centrifuged for 10 min at 1,967 ×g, and the supernatant (PL) was recovered and delivered at a depth of 3 mm into the appropriate treatment zone through a 1 mL Luer lock syringe equipped with a 25-gauge needle.
Assessment of hair growth parameters
For each patient, individual follicular units were counted in each box using a counting incision device (Cole Instruments) loaded with a gentian marker to prevent duplicating the count for any follicular unit and to record a precise number (see Figures 4,5). The number of growing follicular units measuring at least 50 mm were recorded for each of the three regions of interest, that is, the PL, AA–PRP, and saline-treated grafts.
GF quantification
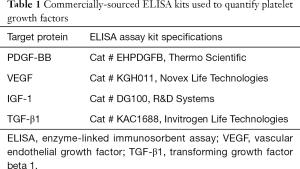
AA–PRP and PL samples intended for GF concentration testing were prepared as described above. For PL samples, the total sonication time was set to 0 (no treatment), 15 min (i.e., 30 on/off cycles), or 30 min (i.e., 60 on/off cycles). All samples were centrifuged for 10 min at 1,967 ×g, and the recovered supernatant was stored at 4 °C prior to testing. The concentrations of four GFs [PDGF-BB, IGF-1, transforming growth factor beta 1 (TGF-β1), and vascular endothelial growth factor (VEGF)] were measured using commercially-sourced enzyme-linked immunosorbent assay (ELISA) kits (Table 1) in accordance with standard, previously reported protocol (14). Briefly, standards and samples were added to a 96-well microplate pre-coated with an antibody against the target GF. An enzyme-linked polyclonal antibody, specific for the target GF was added in excess and the unbound antibody was rinsed away. A substrate solution was then added and color developed in proportion to the quantity of bound GF. After the color development was stopped, the absorbance was measured at 450 nm using a µQuant microplate reader (Bio-Tek). GF concentrations were determined from standard curves with the aid of GraphPad Prism curve-fitting software. For IGF-1 and TGF-β1, the data was linearized by plotting the log(concentration) versus the log(OD450), and a best fit was found via linear regression analysis. For all others, a nonlinear four-parameter logistic curve fit was performed. For Regen-derived GF measurements, n=5. GF measurements for the Arthrex Angel system contained only 2 of the above 5 subjects (n=2).
Full table
Statistical analysis
Platelet GF concentrations are expressed as mean ± standard error (n=5 or n=2). Unpaired t-tests (α =0.05) were used to determine differences in mean among the baseline GF concentrations in A–PRP derived from the Regen and Arthrex systems. One-way Analysis of Variance (ANOVA) was used to determine statistical significance between data sets (α =0.05). For data sets with significant differences in means, the Tukey test was applied to identify the source(s) of variation
Results
The number of visible follicular units within the PL, AA–PRP, and control boxes at follow-up, in addition to the number of follicular units placed within each box during the surgical procedure, are provided in Table 2 for the three primary participants of this study. Transplanted follicular unit growth was highest in those regions treated with PL, where 89%±9% and 99% of follicular units were visible at 14 and 18 weeks, respectively. Although the AA–PRP region appeared more successful than the control at 14 weeks with 74%±7% versus 57%±10% of transplanted grafts visible, there was little disparity between these two groups at 18 weeks (75% versus 72% for AA–PRP and saline treatment zones, respectively).

Full table
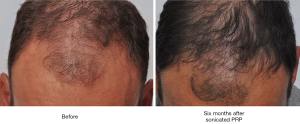
For comparative purposes, the entire scalp of a single subject (Subject D, 44, Norwood Five) was treated exclusively with PL (10 mL). Four tattoos were drawn on the mid-scalp, and hair checks were performed on these regions at 28 weeks post-treatment. At this time, the patient demonstrated an increase in visible follicular unit density and hair density from starting values of 60 FU cm−2, and 90 hair cm−2 to 90 FU cm−2, and 200 hair cm−2. Before and after photos for this patient are shown in Figure 6. As a control, 18 grafts were placed into a scar and treated only with saline. At 16 weeks, only 10 of these grafts were visible.
Concentrations of IGF-1, TGF-β1, PDGF-BB, and VEGF for intact and lysed Arthrex-derived PRP and native and activated Regen-derived PRP are provided in Table 3. No significant difference was observed between native PRP collected via Regen BCT or the Arthrex Angel system for any of the GFs evaluated in this study. Overall, the highest concentrations were obtained from Arthrex PRP samples that underwent a 30 min sonication. One notable exception was the IGF-1 value, as no significant difference in means was found for this GF (P=0.98). For the remaining three GFs (i.e., TGF-β1, VEGF, and PDGF-BB), a 30-min sonication produced significantly higher concentrations than did activation with calcium gluconate, which was not significantly different from native Regen PRP with respect to any GF.

Full table
Discussion
The results of this work show that transplanted grafts resume growth faster in the presence of PL than treatment with AA–PRP or saline. However, for Subject A, it was noted that the quality of grafts in the Regen-derived AA–PRP region was superior to those in the Arthrex-derived PL region upon initial placement and in the follow-up evaluation at 14 weeks. Even though the coverage was aesthetically better in the AA–PRP box, the number of grafts growing was higher in the PL box, a phenomenon likely attributable to the grafts in the Regen box coincidentally containing a greater number of hairs per follicular unit than those placed in the PL box. Nevertheless, the faster rate of regrowth for grafts placed in the presence of PL cannot be disputed. Indeed, the singular, beneficial influence of PL in hair regeneration is best illustrated in Subject D, as this patient was treated exclusively with PL over his entire recipient region; no graft transplantations were performed. As previously noted, the frontal regions of Subjects A, B, and C, were treated with PL, and since vascular flow from the frontal scalp to adjacent regions of the scalp cannot be blocked, the potential for follicular regeneration within the AA–PRP or saline boxes to be positively influenced by PL injected in the frontal scalp could not be avoided entirely. Importantly, the locations of the three test boxes in the recipient region of the scalp were shuffled in an effort to account for any contamination of the results.
A plausible hypothesis for the superior follicular regeneration rate of PL-treated grafts over AA–PRP-treated grafts may be drawn based on the relative GF concentrations in PL and AA–PRP, respectively. With the previously noted exception of IGF-1, which was statistically equivalent for all test groups, PL contained significantly higher GF concentrations than AA–PRP. As suggested by their names, GFs promote cell growth through several pathways. VEGF, for example, promotes angiogenesis, which establishes a conduit for nutrient delivery and waste removal (19), and TGF-β1 prevents basal keratinocytes from reaching terminal differentiation which ensures the proliferative potential of this cell population is retained (20). When dermal papilla cells are cultured in the presence of activated PRP, higher concentrations of molecules linked to hair growth are measured with respect to controls. Specifically, higher levels of β-catenin, the protein in the Wnt pathway that is responsible for stimulating hair follicle development (21,22), have been observed (23). Thus, it is reasonable to expect grafts placed with higher concentrations of platelet-derived GFs to regenerate hair follicles sooner than those placed with lower concentrations of the same molecules.
Given that PL and AA–PRP are derived from identical sources (i.e., platelet concentrated whole blood), one may question why PL contains markedly higher levels of most GFs. The answer likely stems from the mechanism by which platelets relinquish GFs in the respective processes. When platelets are activated by exposure to calcium gluconate, GFs are released from the α-granules and glycoprotein IIb/IIIa, a receptor able to bind fibrinogen, is translocated to the cell membrane (18). Binding of fibrinogen to glycoprotein receptors on adjacent platelets causes them to adhere to one another, and coagulation ensues. Ultimately, a platelet plug is formed, within which a high concentration of GFs is trapped. The rate at which these bioactive proteins can reach surrounding tissues to initiate their respective pathways becomes diffusion- and mesh size-limited. Small proteins should be released from the plug faster than large proteins which may require some degree of plug degradation before they are released. Alternatively, when platelets are lysed, the contents of α-particles are released without aggregation, as the cell walls are no longer intact. In terms of a therapy tool, the limiting factor for concentrating GFs becomes the percentage of platelets that are lysed in a given sonication session.
The relative GF concentrations measured in this study indicate that molecular size does influence recovery in a method dependent manner. IGF-1 (7.6 kDa), a protein that is substantially smaller than TGF-β1 (~25 kDa), PDGF-BB (~30 kDa) or VEGF (~38 kDa) (24-26), was present in statistically equivalent concentrations for all PRP treatments. The remaining GFs, which are very similar in size, exhibited a six- to eight-fold greater concentration in PL originating from a 30-min sonication period than in AA–PRP. Conceivably, these larger proteins were not readily expelled out of the platelet plug upon centrifugation owing to pores within the plug being too small.
The immediate and positive influence of a bolus GF delivery from PL on follicular regeneration has been discussed above; nevertheless, given the upward trend in follicular unit density and hair density when PL is administered as a stand-alone therapy, one may conclude (albeit with limited confidence, n=1, Subject D) that secondary factors are at play. We hypothesize that PL may afford activation of additional platelets and/or initiation of relevant hair stimulation pathways more effectively than AA–PRP since the platelet cell membranes are removed. In PL, molecules that participate in the clotting cascade (i.e., ADP and ATP from dense granules) or that are released to activate additional platelets (i.e., thromboxane A2 from α-granules) are free to diffuse into the surrounding tissue without being bound by platelets within the venous blood draw that were concentrated into the PL precursor, A–PRP. Since the precise cellular mechanisms encompassing the beneficial nature of PL in hair replacement surgeries has yet to be identified, the authors recommend that this field be more thoroughly examined given the promising, side effect-limited nature of the therapy.
Acknowledgements
This work was supported by a grant from the International Society of Hair Restoration Surgery.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Informed consent was obtained from all study patients.
References
- Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med 2002;4:1-11. [Crossref] [PubMed]
- Rogers NE, Avram MR. Medical treatments for male and female pattern hair loss. J Am Acad Dermatol 2008;59:547-66. [Crossref] [PubMed]
- Rousso DE, Kim SW. A review of medical and surgical treatment options for androgenetic alopecia. JAMA Facial Plast Surg 2014;16:444-50. [Crossref] [PubMed]
- Schweiger ES, Boychenko O, Bernstein RM. Update on the pathogenesis, genetics and medical treatment of patterned hair loss. J Drugs Dermatol 2010;9:1412-9. [PubMed]
- Olsen EA, Weiner MS. Topical minoxidil in male pattern baldness: effects of discontinuation of treatment. J Am Acad Dermatol 1987;17:97-101. [Crossref] [PubMed]
- Gupta AK, Carviel JL. Meta-analysis of efficacy of platelet-rich plasma therapy for androgenetic alopecia. J Dermatolog Treat 2017;28:55-8. [Crossref] [PubMed]
- Gentile P, Cole JP, Cole MA, et al. Evaluation of Not-Activated and Activated PRP in Hair Loss Treatment: Role of Growth Factor and Cytokine Concentrations Obtained by Different Collection Systems. Int J Mol Sci 2017;18:E408. [Crossref] [PubMed]
- Takikawa M, Nakamura S, Nakamura S, et al. Enhanced effect of platelet-rich plasma containing a new carrier on hair growth. Dermatol Surg 2011;37:1721-9. [Crossref] [PubMed]
- Gentile P, Garcovich S, Bielli A, et al. The Effect of Platelet-Rich Plasma in Hair Regrowth: A Randomized Placebo-Controlled Trial. Stem Cells Transl Med 2015;4:1317-23. [Crossref] [PubMed]
- Gkini MA, Kouskoukis AE, Tripsianis G, et al. Study of platelet-rich plasma injections in the treatment of androgenetic alopecia through an one-year period. J Cutan Aesthet Surg 2014;7:213-9. [Crossref] [PubMed]
- Cervelli V, Garcovich S, Bielli A, et al. The effect of autologous activated platelet rich plasma (AA-PRP) injection on pattern hair loss: clinical and histomorphometric evaluation. Biomed Res Int 2014;2014:760709. [PubMed]
- Bouhanna P, Bouhanna E. Hair Transplantation for Aesthetic Surgery of the Scalp and Body Hair. In: Bouhanna P, Bouhanna E, editors. Alopecias: Diagnosis and Treatments. Boca Raton: CRC Press-Taylor & Francis Group, 2016:163-88.
- Cole JP. Status of Individual Follicular Group Harvesting. Hair Transplant Forum Int 2009;19:20.
- Jimenez F, Izeta A, Poblet E. Morphometric analysis of the human scalp hair follicle: practical implications for the hair transplant surgeon and hair regeneration studies. Dermatol Surg 2011;37:58-64. [Crossref] [PubMed]
- Zhang H, Tasnim F, Ying JY, et al. The impact of extracellular matrix coatings on the performance of human renal cells applied in bioartificial kidneys. Biomaterials 2009;30:2899-911. [Crossref] [PubMed]
- Rose PT. Hair restoration surgery: challenges and solutions. Clin Cosmet Investig Dermatol 2015;8:361-70. [Crossref] [PubMed]
- Uebel CO, da Silva JB, Cantarelli D, et al. The role of platelet plasma growth factors in male pattern baldness surgery. Plast Reconstr Surg 2006;118:1458-66; discussion 1467. [Crossref] [PubMed]
- Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost 2011;105 Suppl 1:S13-33. [Crossref] [PubMed]
- Pierce GF, Mustoe TA, Lingelbach J, et al. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol 1989;109:429-40. [Crossref] [PubMed]
- Shipley GD, Pittelkow MR, Wille JJ Jr, et al. Reversible inhibition of normal human prokeratinocyte proliferation by type beta transforming growth factor-growth inhibitor in serum-free medium. Cancer Res 1986;46:2068-71. [PubMed]
- Lichtenberger BM, Mastrogiannaki M, Watt FM. Epidermal β-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat Commun 2016;7:10537. [Crossref] [PubMed]
- Andl T, Reddy ST, Gaddapara T, et al. WNT signals are required for the initiation of hair follicle development. Dev Cell 2002;2:643-53. [Crossref] [PubMed]
- Li ZJ, Choi HI, Choi DK, et al. Autologous platelet-rich plasma: a potential therapeutic tool for promoting hair growth. Dermatol Surg 2012;38:1040-6. [Crossref] [PubMed]
- Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem 1978;253:2769-76. [PubMed]
- Neufeld G, Cohen T, Gengrinovitch S, et al. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 1999;13:9-22. [PubMed]
- Assoian RK, Komoriya A, Meyers CA, et al. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem 1983;258:7155-60. [PubMed]
Cite this article as: Cole JP, Cole MA, Insalaco C, Cervelli V, Gentile P. Alopecia and platelet-derived therapies. Stem Cell Investig 2017;4:88.


