Aplastic anemia is related to alterations in T cell receptor signaling
Introduction
Aplastic anemia (AA) is a disease characterized by bone marrow hematopoietic dysfunction and peripheral blood pancytopenia (1). The severity of AA is defined according the blood count parameters and bone marrow findings. Specially, severe aplastic anemia (SAA) patient had a marrow biopsy showing less than 25% of normal cellularity or, marrow showing less than 50% normal cellularity in with fewer than 30% of the cells are hematopoietic and 2/3 of the following: neutrophil count 9/L, platelet count 9/L, or reticulocyte count 9/L. Very severe aplastic anemia (VSAA) patient was further defined by neutrophils 9/L. Patient not meeting the criteria for SAA or VSAA was diagnosed with non-severe aplastic anemia (NSAA) (2). Laboratory and clinical results indicate that AA is a T cell immune-mediated autoimmune disease that involves destruction of hematopoietic progenitor/stem cells (3). This observation is validated by the powerful evidence that treatment with cyclosporine A, prednisolone, and antithymocyte globulin leads to hematological recovery in a majority of patients with AA. Mechanisms of T cell-mediated destruction of hematopoiesis include oligoclonal expanded populations of autologous T cells, which cause a significant decrease in the number of hematopoietic progenitor/stem cells (4), a Th1 polarization response conferring an immoderate production of inhibitory cytokines, and a Th17 immune response (4-6). Regulatory T-cells (Tregs) impairment also plays a critical role in the pathophysiology of AA (7). In that sense, T cells play a key role in the imbalance in immune homeostasis in AA. Moreover, aberrant T cell activation was shown to be involved in the misbalance in T cell immune homeostasis in autoimmune disease where alterations in T cell receptor (TCR) signaling play a crucial role.
TCR signaling and its regulatory factors
TCRs recognize antigens bound to MHC molecules on the surface of antigen-presenting cells (APCs). This event results in the transduction of TCR signals which lead to cell proliferation, differentiation, cytokine secretion, and other effector functions (8). Due to the central role of T cells in the immune system, alterations in TCR signaling can lead to diseases, such as immunodeficiency or autoimmunity.
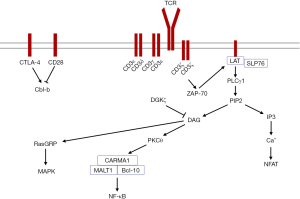
The TCR complex comprises the ligand-binding subunit TCRαβ/γδ and the CD3 complex, which includes the dimers CD3εδ, CD3εγ, or CD3ζζ (9). The TCR has a short intracellular sequence that lacks signaling capability, but after T cell stimulation by an antigen, the immunoreceptor tyrosine-based activating motifs (ITAMs) in the CD3 molecules undergoes phosphorylation, which can then phosphorylate and recruit the downstream signaling molecule zeta-associated protein of 70 kDa (ZAP-70). ZAP-70 mediates the tyrosine phosphorylation of adaptor proteins such as SH2 domain-containing leukocyte protein of 76 kDa (SLP-76) and linker for activation of T cells (LAT). Phosphorylated LAT directly binds phospholipase C-γ1 (PLCγ1), and this protein catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to generate two breakdown products, inositol-1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 triggers increased calcium, which dephosphorylates the transcription factor nuclear factor of activated T cell (NFAT). DAG recruits effector proteins including protein kinase C-θ (PKCθ) and Ras guanyl nucleotide-releasing protein (RasGRP). PKCθ activates the adapter protein complex CARD domain and MAGUK domain-containing protein-1 (CARMA1), B-cell lymphoma 10 (Bcl-10), and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1). This complex promotes activation of nuclear factor kappa B (NF-κB). RasGRP activates mitogen-activated protein kinase (MAPK) pathways (10-13).
Precise T cell activation is controlled by positive (costimulatory) or negative (coinhibitory) signaling molecules (14). After binding to CD80 or CD86 on APCs, the costimulator CD28 interacts with PKCθ to activate NF-κB and sustains antigen-induced T cell activation. The coinhibitory cytotoxic T-lymphocyte associated protein 4 (CTLA-4), which is expressed on activated T cells also binds to CD80 or CD86 and interrupts T cell activation (15,16). After TCR ligation by specific antigens, T cells are stimulated to proliferate and differentiate into the cytotoxic effectors Th1 and Th2 helper cells, regulatory T cells, Th17 cells, or follicular helper T cells, which are influenced by the cytokine milieu and strength and duration of the TCR signal (17,18).
Aberrant TCR signaling in AA
There are only a few reports regarding the role of the TCR signaling pathway in AA pathogenesis. Here, we summarize the clinical and laboratory findings characterizing the role of the TCR signaling pathway in AA.
The characteristics of the CD3 molecules and CD3ζ 3'-UTR isoforms in AA
The CD3 signaling molecules play a central role in the TCR signaling pathway. In most reports, a significant increase in the CD3γ, CD3δ, CD3ε, and CD3ζ expression levels was detected in peripheral blood samples from newly diagnosed AA patients, which may result in enhancing T cell activation (19,20). Interestingly, the CD3ζ expression level was higher in NSAA compared with SAA patients. However, in contrast with these reports, there was a study demonstrating decreased CD3ζ protein in AA patients. This result was derived from only five newly diagnosed patients with AA and 25 AA patients after immunosuppression therapy (21). The discrepancies might be due to different disease statuses.
Within the CD3 complex, CD3ζ is the key signaling molecule in the TCR signaling pathway. Expression of the CD3ζ gene is regulated at the transcriptional, posttranscriptional, and posttranslational levels (22). It has been reported that aberrant CD3ζ expression is associated with different CD3ζ 3'-UTR isoforms. There are two CD3ζ 3′-UTR isoforms: wild type (WT) and an alternatively spliced (AS) isoform (23,24). The distribution of the WT and AS CD3ζ 3'-UTRs in AA patients is different than that in healthy individuals. A significantly lower frequency of the WT+AS+CD3ζ 3'-UTR genotype was detected in AA patients, while a significantly higher frequency of the WT+AS-CD3ζ 3'-UTR and WT−AS+CD3ζ 3'-UTR genotypes was detected in AA patients. Notably, an increased CD3ζ expression level was found in the WT−AS+CD3ζ 3'-UTR individuals compared with SAA patients who had either the WT+AS+CD3ζ 3'-UTR or WT+AS−CD3ζ 3'-UTR genotypes. There was no significant difference in the CD3ζ expression level between the WT+AS+, WT−AS+, and WT+AS− subgroups of NSAA patients (20). Therefore, there may be a difference in the regulation of the CD3ζ expression level and T cell activation between SAA and NSAA patients. At a minimum, it is thought that the CD3ζ 3'-UTR isoforms may influence the CD3ζ expression level in T cells in SAA patients, which may be a cause of the abnormal T cell activation. These results revealed that a posttranslational regulation mechanism was involved in regulating CD3ζ expression in AA. MicroRNAs act as posttranscriptional regulators which specifically pair with complementary sites in 3’-UTRs of target mRNAs to block translation or increase mRNA degradation. Hence, further studies are needed to explore the regulation mechanism of miRNAs that target CD3ζ in AA.
CD28 and CTLA-4 and their related factors in AA
CTLA-4 and CD28 is a pair of homologous receptors expressed on T cells. These proteins share the same ligands while have opposing biological functions. CD28 is an important costimulatory molecule that provides a second signal that permits full T cell activation. Upon T cell activation, CTLA-4 is induced and outcompetes CD28 for ligands, thereby preventing excessive T cell activation (25,26).
Casitas B-lineage lymphoma proto-oncogene-b (Cbl-b), a RING finger E3 ubiquitin-protein ligase, is critical for establishing the threshold for T cell activation (27). Results have shown that CD28 promotes Cbl-b degradation, whereas CTLA-4 promotes Cbl-b re-expression (28). An increased CD28 and decreased CTLA-4 and Cbl-b mRNA expression level were also found in patients newly diagnosed with AA (20). These results suggest that aberrant T cell activation in AA not only associates with the initial signals, but it is also related to the secondary signals in the TCR signaling pathway. Downregulated Cbl-b expression in AA suggests that the threshold for T cell activation is different than that in healthy individuals.
Other TCR signaling molecules and their regulatory factors in AA
As the upstream signaling kinase, ZAP-70 performs a major role in T cell activation, while LAT plays an important role in the recruitment and assembly of multiple signaling effector proteins (29). An increased level of LAT, total phosphorylated LAT, and ZAP-70 were detected in SAA patients. The expression of LAT is positively correlated with the expression of perforin and granzyme B, which are associated with cytotoxic T cell functions. After immunosuppression therapy, the expression of ZAP-70 and LAT dramatically decrease. Inhibition of LAT expression in T cells results in a significant decrease in the expression of perforin and granzyme B and the production of IFN-γ in SAA patients (30). Therefore, increased LAT expression contributes to T cell hyperfunction in AA.
DAG is a critical second messenger that mediates TCR signaling. The abundance of DAG is reduced by DGKs. In T cells, the predominant DGK isoforms are DGKα and DGKζ (31). DGKζ terminates DAG-mediated downstream signals and negatively regulates T cell activation. This finding was confirmed by repressed DGKζ expression, which enhances T cell activation by elevating the miRNA34a level in AA patients (32).
Summary and prospects
Aberrantly expressed TCR signaling molecules, such as upregulated CD3γ, CD3δ, CD3ε, CD3ζ, CD28, ZAP-70, and LAT and downregulated CTLA-4 and Cbl-b may contribute to inappropriate T cell activation in AA patients (Figure 1). There is variation in TCR signaling molecules among AA individuals that may be associated with different disease statuses, genetic backgrounds, and regulatory mechanisms. Moreover, a global profile of molecules related to the TCR signaling pathway characterized at the genetic or epigenetic levels is needed to provide a precise illustration of T cell activation and strategies for targeted immunotherapy in AA.
Acknowledgements
Funding: This study was sponsored by grants from the National Natural Science Foundation of China (81370605 and 81460026), the Natural Science Foundation of Guangdong province (S2012010008794), Science and Information Technology of Guangzhou funded basic research for application project (2014A020212209) and the Fundamental Research Funds for the Central Universities (21612425).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Xu LP, Jin S, Wang SQ, et al. Upfront haploidentical transplant for acquired severe aplastic anemia: registry-based comparison with matched related transplant. J Hematol Oncol 2017;10:25. [Crossref] [PubMed]
- Marsh JC, Ball SE, Cavenagh J, et al. Guidelines for the diagnosis and management of aplastic anaemia. Br J Haematol 2009;147:43-70. [Crossref] [PubMed]
- Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood 2006;108:2509-19. [Crossref] [PubMed]
- Nakao S, Feng X, Sugimori C. Immune pathophysiology of aplastic anemia. Int J Hematol 2005;82:196-200. [Crossref] [PubMed]
- de Latour RP, Visconte V, Takaku T, et al. Th17 immune responses contribute to the pathophysiology of aplastic anemia. Blood 2010;116:4175-84. [Crossref] [PubMed]
- Young NS, Scheinberg P, Calado RT. Aplastic anemia. Curr Opin Hematol 2008;15:162-8. [Crossref] [PubMed]
- Shi J, Ge M, Lu S, et al. Intrinsic impairment of CD4(+)CD25(+) regulatory T cells in acquired aplastic anemia. Blood 2012;120:1624-32. [Crossref] [PubMed]
- Wu W, Yan C, Shi X, et al. Lipid in T-cell receptor transmembrane signaling. Prog Biophys Mol Biol 2015;118:130-8. [Crossref] [PubMed]
- Chakraborty AK, Weiss A. Insights into the initiation of TCR signaling. Nat Immunol 2014;15:798-807. [Crossref] [PubMed]
- Notarangelo LD. Immunodeficiency and immune dysregulation associated with proximal defects of T cell receptor signaling. Curr Opin Immunol 2014;31:97-101. [Crossref] [PubMed]
- Brownlie RJ, Zamoyska R. T cell receptor signalling networks: branched, diversified and bounded. Nat Rev Immunol 2013;13:257-69. [Crossref] [PubMed]
- Li MO, Rudensky AY. T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat Rev Immunol 2016;16:220-33. [Crossref] [PubMed]
- Burbach BJ, Medeiros RB, Mueller KL, et al. T-cell receptor signaling to integrins. Immunol Rev 2007;218:65-81. [Crossref] [PubMed]
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 2013;13:227-42. [Crossref] [PubMed]
- Porciello N, Tuosto L.. CD28 costimulatory signals in T lymphocyte activation: Emerging functions beyond a qualitative and quantitative support to TCR signalling. Cytokine Growth Factor Rev 2016;28:11-9. [Crossref] [PubMed]
- Myint ZW, Goel G. Role of modern immunotherapy in gastrointestinal malignancies: a review of current clinical progress. J Hematol Oncol 2017;10:86. [Crossref] [PubMed]
- Walker LS. EFIS Lecture: Understanding the CTLA-4 checkpoint in the maintenance of immune homeostasis. Immunol Lett 2017;184:43-50. [Crossref] [PubMed]
- Jin Z, Luo Q, Lu S, et al. Oligoclonal expansion of TCR Vδ T cells may be a potential immune biomarker for clinical outcome of acute myeloid leukemia. J Hematol Oncol 2016;9:126. [Crossref] [PubMed]
- Li B, Liu S, Niu Y, et al. Altered expression of the TCR signaling related genes CD3 and FcεRIγ in patients with aplastic anemia. J Hematol Oncol 2012;5:6. [Crossref] [PubMed]
- Li B, Guo L, Zhang Y, et al. Molecular alterations in the TCR signaling pathway in patients with aplastic anemia. J Hematol Oncol 2016;9:32. [Crossref] [PubMed]
- Solomou EE, Wong S, Visconte V, et al. Decreased TCR zeta-chain expression in T cells from patients with acquired aplastic anaemia. Br J Haematol 2007;138:72-6. [Crossref] [PubMed]
- Clevers H, Alarcon B, Wileman T, et al. The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol 1988;6:629-62. [Crossref] [PubMed]
- Tsuzaka K, Nozaki K, Kumazawa C, et al. TCRzeta mRNA splice variant forms observed in the peripheral blood T cells from systemic lupus erythematosus patients. Springer Semin Immunopathol 2006;28:185-93. [Crossref] [PubMed]
- Nambiar MP, Enyedy EJ, Warke VG, et al. T cell signaling abnormalities in systemic lupus erythematosus are associated with increased mutations/polymorphisms and splice variants of T cell receptor zeta chain messenger RNA. Arthritis Rheum 2001;44:1336-50. [Crossref] [PubMed]
- Gardner D, Jeffery LE, Sansom DM. Understanding the CD28/CTLA-4 (CD152) pathway and its implications for costimulatory blockade. Am J Transplant 2014;14:1985-91. [Crossref] [PubMed]
- Callahan MK, Postow MA, Wolchok JD. CTLA-4 and PD-1 Pathway Blockade: Combinations in the Clinic. Front Oncol 2015;4:385. [Crossref] [PubMed]
- Paolino M, Thien CB, Gruber T, et al. Essential role of E3 ubiquitin ligase activity in Cbl-b-regulated T cell functions. J Immunol 2011;186:2138-47. [Crossref] [PubMed]
- Li D, Gál I, Vermes C, et al. Cutting edge: Cbl-b: one of the key molecules tuning CD28- and CTLA-4-mediated T cell costimulation. J Immunol 2004;173:7135-9. [Crossref] [PubMed]
- Billadeau DD. T cell activation at the immunological synapse: vesicles emerge for LATer signaling. Sci Signal 2010;3:pe16. [Crossref] [PubMed]
- Sheng W, Liu C, Fu R, et al. Abnormalities of quantities and functions of linker for activations of T cells in severe aplastic anemia. Eur J Haematol 2014;93:214-23. [Crossref] [PubMed]
- Andrada E, Almena M, de Guinoa JS, et al. Diacylglycerol kinase ζ limits the polarized recruitment of diacylglycerol-enriched organelles to the immune synapse in T cells. Sci Signal 2016;9:ra127. [Crossref] [PubMed]
- Sun YX, Li H, Feng Q, et al. Dysregulated miR34a/diacylglycerol kinase ζ interaction enhances T-cell activation in acquired aplastic anemia. Oncotarget 2017;8:6142-54. [PubMed]
Cite this article as: Xiao Y, Zhao S, Li B. Aplastic anemia is related to alterations in T cell receptor signaling. Stem Cell Investig 2017;4:85.


