Graft predominance after double umbilical cord blood transplantation: a review
Introduction
Double umbilical cord blood transplantation (dUCBT) was developed in order to increase the cell dose and to allow adult patients without a sufficiently sized single cord blood unit (CBU) to proceed to that type of transplantation (1). It appeared to be associated with less graft failure in adult patients, but with similar recovery kinetics of leucocytes and platelets as compared to single UCBT (1-3). Importantly, hematopoietic recovery after dUCBT generally originates from only a single cord blood unit, designated as the predominant (PD)-CBU (1-3). Single donor chimerism is readily documented within 3 months post-transplant in the majority of patients (1-5), but actually may occur as early as 7–14 days after transplantation (6-9). Graft predominance was earlier suggested to be T-cell mediated, as higher doses of graft CD3+ T-cells and also early recovery of alloreactive, IFN-gamma secreting CD8+ T-cells appeared associated with graft predominance (5,10,11). Following our earlier observations that blood CD4+ T-cell numbers rapidly increase after dUCBT (8) and early CD4+ T-cell chimerism predicts for graft predominance (6), we hypothesized that alloreactive HLA-class II-specific CD4+ T-cells from the ‘winning’ CBU may contribute to rejection of the ‘loser’ CBU. That hypothesis was confirmed in a recent study from our group, showing that HLA-class II allele-specific CD4+ T-cells were readily detectable after transplantation and were able to recognize both class II mismatched cord blood progenitor cells and primary leukemic cells, which was associated with T-cell effector function (12). This study heavily depended on the use of a unique set of laboratory tools, including HLA-class II allele transduced HELA-cells, by which very low numbers of alloreactive T-cells could be propagated (13) and subsequently be evaluated for effector function towards class II mismatched hematopoietic cells and primary leukemic cells. A library of stimulator cells expressing a single HLA-class II allele was generated by retroviral transduction of HLA-class II negative HeLa cells with different combinations of HLA-DRA1/B1-5, HLA-DQA1/B1 or HLA-DPA1/B1 molecules, as described (14). Propagated T-cells were co-cultured with HeLa cells transduced with a specific mismatched HLA-class II molecule derived from the non-engrafting (NE)-CBU, followed by assessment of T-cell activation antigens and effector markers. Here we present an individual patient example as well as a summary of results in the context of the most recent literature.
Results and discussion
Results of an individual patient
Post dUCBT peripheral blood mononuclear cells (PBMC) were first propagated by HLA-class II allele-specific stimulation in co-culture with irradiated HeLa cells transduced with a single HLA-class II (mismatched, or 3rd party neither expressed by the PD-NE nor recipient) molecule (14–21 days), followed by analysis of the T-cell culture for alloreactivity. To analyze T-cell alloreactivity, the propagated T-cells were co-cultured for 2–20 h with HeLa cells transduced with the selected mismatched, matched or 3rd party HLA-class II alleles followed by FCM assessment of measures of specific immune reactivity in propagated T-cells, typically being upregulation of the activation marker CD137. Figure 1 shows detailed results of patient UPN 02 showing 14% CD137+/CD4+ T-cells reactive with the DRB1*01.01 mismatch versus 1.6% CD137+/CD4+ T-cells with specificity for the matched DRB1+03.01 allele.
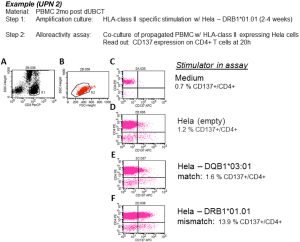
Mismatched HLA-class II-specific amplification—alloreactivity assay set up and gating strategy. Patient 2, PBMC from 2 mo post dUCBT were subjected to HLA-class II allele-specific amplification using HeLa cells transduced with mismatched matched HLA-class II alleles DRB1*01.01, and subsequently assayed for HLA-class II allele specific upregulation of CD137 by FCM after stimulation with HeLa cell lines transduced with the (mis)matched alleles. Analysis was performed by using a sequential gating strategy, i.e., Step 1: gating on T-cells on basis of their CD3+, SSClow characteristics (gate G1 = plot A), excluding stimulator HeLa cells; Step 2: gating on viable cells (FCS/SSC scatter [gate G2 (=region R2, events fulfilling the criteria of regions R1 and R2; plot B]; Step 3: the events in Gate 2 are plotted in a CD4/PE vs. CD137/APC plot and applying quadrant statistics. As controls responder cells were incubated with ‘empty’ stimulator HeLa cells, HeLa cells transduced with a matched HLA-class II allele or with HeLa cells transduced with an unrelated (third party, not shown) HLA-class II allele. Inserted numbers represent the proportions of CD4+ T-cells expressing the activation/effector markers. PBMC, peripheral blood mononuclear cells; dUCBT, double umbilical cord blood transplantation.
Summary of earlier reported results
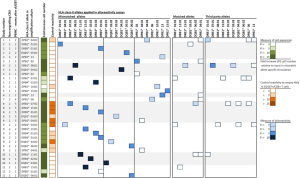
As earlier reported (12), 19 post dUCBT PBMC samples were analyzed for HLA-class II allele-specific T-cell alloreactivity. The HLA-class II allele-specific propagation of the post dUCBT PBMC samples increased T-cell numbers 29 folds (median; range 2 to 787) and further skewed post dUBCT blood T-cells towards CD4+. Results of the alloreactivity assays of the 19 post dUCBT PBMC samples taken from the 11 patients are summarized in Figure 2. In all 11 patients, alloreactive CD4+ T-cells towards one or more HLA-class II mismatched alleles of the NE-CBU were detectable. In total, CD4+ T-cell alloreactivity towards 29 out of 33 (88%) mismatched alleles was detectable, including 15 out of 16 (94%) for DR, 7 out of 7 (100%) for DQ and 7 out of 10 (70%) for DP alleles. CD8+ T-cell alloreactivity towards transduced HeLa cells was present as well, albeit less prominent than CD4+ T-cell alloreactivity. Notably, the highest CD4+ T-cell alloreactive responses were observed in samples taken at 1 month post UCBT. We also showed that allo-reactive CD4+ T-cells were able to exert additional effector T-cell functions, by demonstrating T-cell activation markers (CD134, CD137) as well as effector markers (IFN-γ, CD107). In addition, we could also demonstrate that these alloreactive CD4+ T-cells recognize primary leukemic cells via mismatched HLA-class II alleles shared between the NE-CBU and leukemic cells. Primary leukemic cells of 5 out of 6 patients were recognized by the HLA-class II alloreactive CD4+ T-cells at one or two alleles, whereas irrelevant combinations were negative.
Double UCBT was developed to allow also adult patients, for whom no sufficiently sized single unit could be identified, to proceed to this type of alternative donor transplantation (1). While a lower risk of graft failure was observed after double UCBT, hematopoietic recovery most times resulted from only one unit, whereby recovery kinetics for the various hematopoietic lineages was not significantly different as compared to recovery after single unit UCBT (having engrafted effectively) (1-3). Unit predominance observed by different studies was long poorly understood and parameters predicting for predominance were questioned and addressed in a number of studies. Parameters that appeared to be associated with predominance included the number of CD3+ T-cells in the graft (pre-transplant) and the recovery of CD8+ T-cells after transplantation with specific activity towards the rejected unit (5,10,11). In addition, experimental studies supported a possible causative role of lymphocytes of the predominant unit (15-17). Meanwhile, no evidence could be obtained for a causative role of NK-cells and KIR-mismatching (18-20).
We focused on alloreactive CD4+ T-cells based on the earlier observation that CD4+ T-cells expand early after dUCBT and that CD4+ T-cell chimerism predicts for ultimate unit predominance (6,8). Our observations firmly supported the hypothesis that these alloreactive CD4+ T-cells play a pivotal role in graft predominance after dUCBT. We showed an early in vivo expansion of the mismatch-specific alloreactive CD4+ T-cell pool and a decline following elimination of the non-engrafting unit (NE-CBU). Our findings were dominated by CD4+ T-cell responses, yet CD8+ T-cells did show alloreactivity as well, albeit to a much lower extent (12). Of note, our in vitro expansion protocols may have favored CD4+ T-cells over CD8+ T-cells as a result of the use of HLA-class II antigens for expansion, which may explain the difference with earlier observations by Gutman et al. (10), who identified alloreactive IFN-γ secreting CD8+ T-cells post dUCBT PBMC. The alloreactive CD4+ T-cells we identified, were armored with T-cell effector mechanisms (degranulation and IFN-γ production), compatible with an immediate and targeted (cytotoxic) immune response, which strongly suggest that CD4+ T-cells are the major player in graft-versus-graft alloreactivity.
Interestingly, the non-dominant CBU total nucleated cell (TCN) dose may also play an important role. Purtill et al. (21) showed that the non-dominant-CBU TNC dose was significantly associated with neutrophil engraftment, which might be explained by effective presentation of mismatched HLA-antigens by myeloid precursor cells of the non-dominant CBU, which strongly express both class I and class II antigens. T-cell alloreactivity towards these precursor cells may result in unit predominance but also into enhanced graft versus leukemia (GVL) (22-24). Interestingly, in adult patients suffering from hematological malignancies, dUCBT may be associated with a lower incidence of relapse (25-29). A possible stronger graft versus leukemia (GvL) effect might be associated with a stronger T-cell mediated GVL reactivity, due to multiple major HLA-antigen mismatches (14,29). While alloreactivity towards non-inherited maternal antigens (NIMAs) (30) or inherited paternal antigens (IPAs) (31) also might contribute and also NK-cells and KIR-mismatching might also be involved, the impact of allele mismatching, especially class II mismatching may argue in favor of a T-cell mediated effect (14,20,32,33).
The alloreactive T-cells in our study also recognized leukemic cells in a subset of patients, when the mismatched HLA-class II alleles were shared between NE-CBU and the leukemic cells (12). Thus, a CD4+ T-cell mediated graft-versus-graft alloreactivity may involve and enhance GvL in the dUCBT setting and thereby provide an explanation for the low relapse rate associated with dUCBT. These results are in line with earlier results by Stevanovic et al. (34), showing experimentally that alloreactive CD4+ T-cells directed against mismatched HLA-class II molecules are capable of directly eliminating leukemic cells. In order to exploit this phenomenon in dUCBT, the two CBUs might be selected for reciprocal HLA-class II mismatches that match with the recipient. In this setting the generated graft-versus-graft HLA-class II alloreactivity may reduce relapse incidence and improve clinical outcome by specific targeting of HLA-class II molecules expressed by leukemic cells.
Taken together, results by Stevanovic et al. (34) and our results suggest that graft-versus-graft alloreactivity might potentially facilitate GvL after dUCBT. Despite documented graft-versus-graft reactions in dUCBT, also single unit UCBT has been associated with a low relapse rate and strong GVL-effect, which might be explained by a greater HLA-disparity between donor and recipient when compared with unrelated donor transplantation. Of note, there was no significant difference in relapse rate among children and young adolescents after single- and double-UCBT in two randomized controlled trials (35,36), while dUCBT was suggested to be associated with a higher incidence of graft versus host disease (35). Collectively, these and other (26) observations suggesting a strong GVL-effect after single and double UCBT indicate that single UCBT should be preferred when a cord blood unit with a sufficient dose of progenitor cells is available. Further development is, however, needed by approaches that may enhance engraftment and recover by for example stem cell expansion while preserving alloreactive potential towards the underlying malignancy.
Anti-HLA-class II allele-specific immune reactivity in early post dUCBT peripheral blood samples. PBMC samples obtained from 11 patients at 1 to 5 months after dUCBT were propagated towards mismatched HLA-class II alleles of the NE-CBU (column: HLA-class II allele in amplification culture) in a T cell—Hela cell co-culture. T cell expansion of individual cultures at day 21 in presented as Fold increase cell number. Propagated T cells were subsequently tested for alloreactivity towards mismatched, matched and third party HLA-class II alleles (see blocks: HLA-class II alleles in alloreactivity assays). The response was quantified by CD137 upregulation on CD4+ T-cells and presented as ‘Fold increase (FI) [%CD137+/CD4+]’ of reactivity towards HeLa cells transduced with (mis)matched or third party HLA-class II alleles relative to reactivity towards the not-transduced ‘empty’ HeLa cell (Control reactivity); see also Figure 1. The level of T cell expansion, Control reactivity (towards ‘empty’ Hela cells) and alloreactivity response is presented in a 4-color-grading scale. PBMC, peripheral blood mononuclear cells; dUCBT, double umbilical cord blood transplantation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 2005;105:1343-7. [Crossref] [PubMed]
- Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant 2007;13:82-9. [Crossref] [PubMed]
- Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood 2007;110:3064-70. [Crossref] [PubMed]
- Avery S, Shi W, Lubin M, et al. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood 2011;117:3277-85. [Crossref] [PubMed]
- Ramirez P, Wagner JE, DeFor TE, et al. Factors predicting single-unit predominance after double umbilical cord blood transplantation. Bone Marrow Transplant 2012;47:799-803. [Crossref] [PubMed]
- Somers JA, Braakman E, van der Holt B, et al. Rapid induction of single donor chimerism after double umbilical cord blood transplantation preceded by reduced intensity conditioning: results of the HOVON 106 phase II study. Haematologica 2014;99:1753-61. [Crossref] [PubMed]
- Kang HJ, Kho SH, Jang MK, et al. Early engraftment kinetics of two units cord blood transplantation. Bone Marrow Transplant 2006;38:197-201. [Crossref] [PubMed]
- Somers JA, Brand A, van Hensbergen Y, et al. Double umbilical cord blood transplantation: a study of early engraftment kinetics in leukocyte subsets using HLA-specific monoclonal antibodies. Biol Blood Marrow Transplant 2013;19:266-73. [Crossref] [PubMed]
- Newell LF, Milano F, Nicoud IB, et al. Early CD3 peripheral blood chimerism predicts the long-term engrafting unit following myeloablative double-cord blood transplantation. Biol Blood Marrow Transplant 2012;18:1243-9. [Crossref] [PubMed]
- Gutman JA, Turtle CJ, Manley TJ, et al. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T-cell response against the nonengrafted unit. Blood 2010;115:757-65. [Crossref] [PubMed]
- Milano F, Heimfeld S, Gooley T, et al. Correlation of infused CD3+CD8+ cells with single-donor dominance after double-unit cord blood transplantation. Biol Blood Marrow Transplant 2013;19:156-60. [Crossref] [PubMed]
- Lamers CH, Wijers R, van Bergen CA, et al. CD4+ T-cell alloreactivity toward mismatched HLA class II alleles early after double umbilical cord blood transplantation. Blood 2016;128:2165-74. [Crossref] [PubMed]
- Lamers CH, Willemsen R, van Elzakker P, et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood 2011;117:72-82. [Crossref] [PubMed]
- Rutten CE, van Luxemburg-Heijs SA, Halkes CJ, et al. Patient HLA-DP-specific CD4+ T cells from HLA-DPB1-mismatched donor lymphocyte infusion can induce graft-versus-leukemia reactivity in the presence or absence of graft-versus-host disease. Biol Blood Marrow Transplant 2013;19:40-8. [Crossref] [PubMed]
- Moretta A, Andriolo G, Lisini D, et al. In vitro evaluation of graft-versus-graft alloreactivity as a tool to identify the predominant cord blood unit before double cord blood transplantation. Biol Blood Marrow Transplant 2012;18:1108-18. [Crossref] [PubMed]
- Yahata T, Ando K, Miyatake H, et al. Competitive repopulation assay of two gene-marked cord blood units in NOD/SCID/gammac(null) mice. Mol Ther 2004;10:882-91. [Crossref] [PubMed]
- Eldjerou LK, Chaudhury S, Arcila MHME, et al. Graft-Vs-Graft Immune Interaction Is the Likely Mechanism of Absolute Unit Dominance in Double Unit Cord Blood (DCB) Transplantation Using Patient DCB Grafts: An in Vivo but Not in Vitro Phenomenon. Blood 2008;112:3486.
- Tarek N, Gallagher MM, Chou JF, et al. KIR and HLA genotypes have no identifiable role in single-unit dominance following double-unit umbilical cord blood transplantation. Bone Marrow Transplant 2015;50:150-2. [Crossref] [PubMed]
- Rocha V, Ruggeri A, Spellman S, et al. Killer Cell Immunoglobulin-Like Receptor-Ligand Matching and Outcomes after Unrelated Cord Blood Transplantation in Acute Myeloid Leukemia. Biol Blood Marrow Transplant 2016;22:1284-9. [Crossref] [PubMed]
- Rettman P, Malard F, Legrand N, et al. Impact of KIR/HLA genetic combinations on double umbilical cord blood transplantation outcomes. Results of a French multicentric retrospective study on behalf of the Societe Francophone de Greffe de Moelle et de Therapie Cellulaire (SFGM-TC) and the Societe Francophone d'Histocompatibilite et d'Immunogenetique (SFHI). Bone Marrow Transplant 2016;51:1499-503. [Crossref] [PubMed]
- Purtill D, Stevens CE, Lubin M, et al. Association between Nondominant Unit Total Nucleated Cell Dose and Engraftment in Myeloablative Double-Unit Cord Blood Transplantation. Biol Blood Marrow Transplant 2015;21:1981-4. [Crossref] [PubMed]
- Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood 2009;114:4293-9. [Crossref] [PubMed]
- Kindwall-Keller TL, Hegerfeldt Y, Meyerson HJ, et al. Prospective study of one- vs two-unit umbilical cord blood transplantation following reduced intensity conditioning in adults with hematological malignancies. Bone Marrow Transplant 2012;47:924-33. [Crossref] [PubMed]
- Rodrigues CA, Sanz G, Brunstein CG, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol 2009;27:256-63. [Crossref] [PubMed]
- Ponce DM, Hilden P, Devlin SM, et al. High Disease-Free Survival with Enhanced Protection against Relapse after Double-Unit Cord Blood Transplantation When Compared with T Cell-Depleted Unrelated Donor Transplantation in Patients with Acute Leukemia and Chronic Myelogenous Leukemia. Biol Blood Marrow Transplant 2015;21:1985-93. [Crossref] [PubMed]
- Milano F, Gooley T, Wood B, et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. N Engl J Med 2016;375:944-53. [Crossref] [PubMed]
- Kekre N, Antin JH. Cord blood versus haploidentical stem cell transplantation for hematological malignancies. Semin Hematol 2016;53:98-102. [Crossref] [PubMed]
- Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood 2010;116:4693-9. [Crossref] [PubMed]
- Brunstein CG, Petersdorf EW, DeFor TE, et al. Impact of Allele-Level HLA Mismatch on Outcomes in Recipients of Double Umbilical Cord Blood Transplantation. Biol Blood Marrow Transplant 2016;22:487-92. [Crossref] [PubMed]
- van Rood JJ, Stevens CE, Smits J, et al. Reexposure of cord blood to noninherited maternal HLA antigens improves transplant outcome in hematological malignancies. Proc Natl Acad Sci U S A 2009;106:19952-7. [Crossref] [PubMed]
- van Rood JJ, Scaradavou A, Stevens CE. Indirect evidence that maternal microchimerism in cord blood mediates a graft-versus-leukemia effect in cord blood transplantation. Proc Natl Acad Sci U S A 2012;109:2509-14. [Crossref] [PubMed]
- Willemze R, Rodrigues CA, Labopin M, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia 2009;23:492-500. [Crossref] [PubMed]
- Kloosterboer FM, van Luxemburg-Heijs SA, Willemze R, et al. Umbilical cord blood-naive T cells but not adult blood-naive T cells require HLA class II on antigen-presenting cells for allo-immune activation. Hum Immunol 2004;65:328-39. [Crossref] [PubMed]
- Stevanovic S, Griffioen M, Nijmeijer BA, et al. Human allo-reactive CD4+ T cells as strong mediators of anti-tumor immunity in NOD/scid mice engrafted with human acute lymphoblastic leukemia. Leukemia 2012;26:312-22. [Crossref] [PubMed]
- Wagner JE Jr, Eapen M, Carter S, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med 2014;371:1685-94. [Crossref] [PubMed]
- Michel G, Galambrun C, Sirvent A, et al. Single- vs double-unit cord blood transplantation for children and young adults with acute leukemia or myelodysplastic syndrome. Blood 2016;127:3450-7. [Crossref] [PubMed]
Cite this article as: Cornelissen JJ, Kalin B, Lamers CH. Graft predominance after double umbilical cord blood transplantation: a review. Stem Cell Investig 2017;4:47.


