From patient centered risk factors to comprehensive prognostic models: a suggested framework for outcome prediction in umbilical cord blood transplantation
Introduction
Unrelated umbilical cord blood transplantation (UCBT) is an established alternative for transplantation from conventional sources of hematopoietic stem cells. Cord blood has several noteworthy benefits (1). First, human leukocyte antigen (HLA) matching between donor and recipient is more permissive compared to other sources (i.e., mobilized peripheral blood, bone marrow), enabling transplantation in patients without a suitable HLA-matched donor- a major issue in minorities (2). Second, the process of stem cell collection is risk-free to mother and donor. Furthermore, the graft is readily available for use when in need. Third, there is a low likelihood of infection transmission [i.e., cytomegalovirus (CMV)] from donor to the recipient. Fourth, cord blood has reduced alloreactivity, resulting in lower rates of acute and chronic graft versus host disease (GVHD) compared with recipients of unrelated donor peripheral blood or bone marrow hematopoietic stem cell transplantation (HSCT) (3,4). That being said, limitations of UCBT include the small number of cells available in cord blood units (CBUs); thus, subjecting patients to an increased risk of graft failure and warranting use of two CBUs, when cell dose criteria are not met (5,6). Furthermore, the kinetics of immune reconstitution are rather slow, leading to an increased risk of infection surrounding the transplantation (7-9). Finally, the stem cell reservoir is restricted to cells harvested at the donor’s birth. Repeated collections for boosting of donor-derived immunity through donor lymphocyte infusion are not feasible.
The challenges of UCBT in adults are unique; an increasing comorbidity burden, adequate cell dosing, and potential for increased toxicity, all come into play. Indeed, outcomes of adults following UCBT were initially poor. Possible causes include the long interval from diagnosis to transplantation in early studies; referral to UCBT as a last resort after a prolonged, unsuccessful matched unrelated search; an advanced disease status at transplantation after multiple treatments, and a lower dose of stem cells compared to contemporary practice (1,10). These factors contributed to the high risk of transplantation-related mortality (TRM) and poor event-free survival (EFS) (11,12). Improvement in supportive care, the introduction of a variety of conditioning regimens, realization that a higher infused cell dose and a greater degree of HLA matching are associated with better survival have all led to better outcomes following UCBT (10).
Prospectively identifying patient who will gain maximal benefit from UCBT with minimal risk is a desirable unmet need. A complex network of parameters related to patient, disease, donor, and procedure all come into play (Table 1). While the role of the last three has been extensively reviewed (1,10,13,14), patient-related risk factors have not received such attention. We, therefore, provide an overview of the prognostic impact of pre-transplant patients’ characteristics in adults undergoing UCBT. Furthermore, since comprehensive prediction models are lacking in UCBT, we suggest a framework for integrating patients’ features among other properties, for their development. Such tools could contribute to patient selection, optimization of the different modifiable features in the process of UCBT (e.g., conditioning regimens, CBU selection), analysis of retrospective data, and design of interventions.

Full table
Baseline evaluation for UCBT
Candidates for UCBT should be subjected to a routine pre-transplant evaluation, similar to the one suggested by Hamadani et al. focusing on the patient, disease, and donor (15). Apart from a detailed history and a complete physical examination, all patients and donors should be screened for infectious agents (e.g., human immunodeficiency virus, hepatitis B, and hepatitis C). Recipients should also be tested for Epstein-Barr virus (EBV) and CMV. ABO typing for recipient and donor is mandatory as well. Also, pulmonary function tests and evaluation left ventricular ejection fraction by two-dimensional echocardiography or multi-gated acquisition scan, needs to be performed. Arrhythmias and conduction abnormalities can be identified by a standard electrocardiogram. The information gathered should be combined with a baseline metabolic profile and complete blood count and used for documentation of comorbidities (16).
With regard to disease, the diagnosis must be confirmed, and remission status accurately determined. Minimal residual disease (MRD) should be measured when indicated. Some data are showing a benefit of UCBT over other graft sources in MRD-positive patients (17). The process of selecting umbilical CBUs for transplantation is beyond the scope of this review. However, data suggests that high-resolution HLA typing of (HLA-A, -B, -C, and -DRB1) be performed for candidate units (18,19).
Patient related features
Age
Similar to allogeneic HSCT, strict age thresholds for performing UCBT are becoming fuzzy. Patients as old as 82 years have been transplanted (20). While initial experience with UCBT was disappointing, with 40% of patients dying before day 100 (11), much has changed. Survival following UCBT in adults is constantly improving (10). Nonetheless, older age remains an important determinant of outcomes. In a pooled analysis of 514 patients with hematological malignancies receiving a single CBU after myeloablative conditioning, older age and advanced disease were predictors of worse survival (21). The hazard ratio (HR) for 1-year overall survival (OS) of ages 40–59 years vs. 12–18 years was 2.43 [95% confidence interval (CI): 1.31–4.52]. Similar findings were reported by Baron et al., in AML patients reserving a single CBU transplantation (22). In two joint studies by EUROCORD and the European Society for Blood and Marrow Transplantation (EBMT) age was associated with increased risk for TRM; Tucunduva et al. showed that acute lymphoblastic leukemia (ALL) patients, older than 34 years, had a HR of 1.9 (95% CI: 1.4–2.6) for TRM, compared to their younger counterparts (23). Similar results were reported by Ruggeri et al., in a cohort of acute myeloid leukemia (AML) and ALL patients (24). In another retrospective analysis of Japanese ALL patients, an age of 51 years or older had a HR of 1.89 (95% CI: 1.30–2.75) for overall mortality, compared to the younger age group (25). These three studies had a heterogeneous population with regard to conditioning regimens and number of CBUs.
The role of age as a prognostic factor is not ubiquitous. In a prospective phase II multicenter trial, AML patients receiving reduced-intensity conditioning (RIC) as part of the UCBT, age was not associated with greater mortality (26). In a cohort of myelodysplastic syndrome (MDS) patients undergoing UCBT, an age of 43 years or more did not correspond with increased risk for TRM or decreased risk for EFS (27). Finally, in a joint analysis of 104 adult lymphoma patients by the Eurocord-Netcord and Lymphoma Working Party of the EBMT, an age of 41 years or greater corresponded with increased risk of acute GVHD following UCBT (HR of 2.92; 95% CI: 1.20–7.13), but not TRM or OS (28). Since older patients are more likely to have an advanced disease status, receive RIC, and to be transplanted with a double CBU, isolating the effect of age is a challenge. Markers of biological age rather than chronological age could potentially improve our ability to predict the course following UCBT.
Performance status
Pre-transplantation performance status is typically evaluated with the Karnofsky performance status (KPS) or Eastern Cooperative Oncology Group (ECOG) scales. Multiple retrospective analyses have demonstrated worse outcomes following allogeneic HSCT in patients with a reduced performance studies (29-32). However, prospective studies tend to exclude patients with low functional capacity. This is especially true in UCBT (12,26,33). Moreover, only a few studies evaluated the role of performance status in cord blood recipients. Brunstein et al., analyzed risk factors for outcomes after RIC UCBT among 110 adults with a variety of malignant hematological disorders (34). A composite of high-risk clinical features, which includes poor organ function, prior fungal infection, and a KPS ≤60 and extensive prior therapy, were associated with increased risk of EFS and decreased OS [HR of 2.2 (1.3–3.8) and 3.0 (1.7–5.2)], respectively. However, since only three patients were in fact with a low KPS, the actual impact of the performance status could not be isolated. In another retrospective single center analysis of 70 patients aged 55 years and older with hematologic disease receiving RIC UCBT, no association was found between the ECOG performance status and OS or TRM (35). Determining the prognostic and therapeutic role of performance status evaluation is challenging; lack of evidence-based literature and the subjective nature of measurement scales limit our ability to define its part of in answering prognostic and therapeutic questions. Nonetheless, performance status quantification likely mirrors how a physician perceives the physiological reserve of a UCBT candidate, and therefore should be documented.
Comorbidities
Documenting patients’ comorbidities is a routine stage when taking a medical history from UCBT candidates. Such information could potentially be useful in assessing procedural risk and in treatment personalization. Data on the role of specific comorbidities in UCBT recipients are lacking. To date, the Hematopoietic Cell Transplant-Co-morbidity Index (HCT-CI) has become the leading tool for quantifying the comorbidity burden in allogeneic HSCT candidates (36). The index assigns weights to 14 medical conditions; the sum of the score is translated to a probabilistic estimation of typical transplantation outcomes (e.g., TRM, survival). The utility and accuracy of the HCT-CI score have been debated. While, some have validated the index in various settings of allogeneic HSCT, others have not found predictive merit (37,38). In retrospective analyses involving a mix of donor types, including cord blood, Majhail et al., and others have shown that the HCT-CI was predictive of TRM and OS (39,40). In contrast, Nakaya et al. and DeFor et al., could not find a similar pattern (41,42).
Only a few studies explored the prognostic role of the comorbidity index in restricted populations of cord blood recipients (Table 2). In a retrospective analysis of 70 patients undergoing a UCBT owing to a variety of hematological disorders, Uchida et al., did not find any association with increasing HCT-CI scores and OS or TRM. However, the cohort was rather small (n=70) and outdated, since transplantations were performed in the years 2002–2005 (35). Gerds et al., studied outcomes of UCBT in MDS patients (n=176) (44). The HCT-CI was considered as one of the covariates in the analysis. The median age was 56 (range, 18–73) and 34% of the patients had an HCT-CI equal or greater to 3. Compared to comorbidity free patients, only a high comorbidity burden (HCT-CI ≥3) was associated with increased TRM and OS risk (HR of 2.40 and 2.22, respectively). Scores of 1–2 did not meet statistical significance for the same endpoints. Salit et al. have also studied the HCT-CI but in patients with various hematological disorders (n=15) (45). The median age and HCT-CI score were 54 (range, 16–73) and 3 (range, 0–8). Patients were stratified to HCT-CI scores of 0–3 vs. >3. In a univariate analysis, the high scoring group had an increased probability of 3-year TRM 50% (95% CI: 30–67) vs. 26% (95% CI: 17–36), P=0.01, and a trend towards decreased 3-year OS 29% (95% CI: 12–48) vs. 40% (95% CI: 27–51), P=0.08. In the multivariate analysis, adjusting for additional covariates, only scores of over three were associated with 3-year TRM (HR of 2.12; 95% CI: 1.11–4.01, P=0.02). Overall, there is a clear indication that patients with a high comorbidity burden are at increased risk for TRM following UCBT; however, the HCT-CI might lack discrimination, since patients with intermediate HCT-CI scores are not segregated from comorbidity free patients. Despite the absence of prospective randomized trials, it is our opinion the type and burden of comorbidities should be taken into consideration when choosing the donor, intensity of conditioning and GVHD prophylaxis regimens.
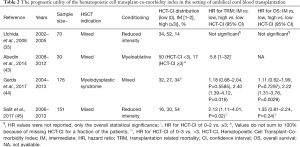
Full table
The prognostic utility of the hematopoietic cell transplant-co-morbidity index in the setting of umbilical cord blood transplantation
¶, HR values were not reported, only the overall statistical significance; ⌇, HR for HCT-CI of 0–2 vs. ≥3; ∆, Values do not sum to 100% because of missing HCT-CI for a fraction of the patients; ⍦, HR for HCT-CI of 0–3 vs. >3. HCT-CI, Hematopoietic Cell Transplant-Co-morbidity Index; IM, intermediate; HR, hazard ratio; TRM, transplantation related mortality; CI, confidence interval; OS, overall survival; NA, not available.
CMV and EBV status
The development of CMV infection before allogeneic transplantation is associated with increased mortality following allogeneic HSCT (31,46,47). Patients undergoing UCBT might be especially prone to CMV reactivation, owing to the slower kinetics of lymphocyte reconstitution (9), compared to alternative donors (48). Therefore, candidates are screened for CMV and monitored for reactivation following transplantation. Testing maternal CMV serology of cord blood donors is not warranted (49). The prognostic role of pre-transplantation CMV serology has been mainly studied in analyses including children with or without adults. Beck et al., found no impact of the pre-transplant CMV serostatus on OS or TRM in a cohort of pediatric and adult UCBT patients. Furthermore, there was not interaction with anti-thymocyte globulin (ATG) administration. However, the 13.8% of patients who did develop CMV disease had significantly higher TRM and lower OS compared to their counterparts (9). In a different population with an age range of 12–55 years, receiving a myeloablative conditioning regimen UCBT, pre-transplant CMV serostatus was associated with an odds ratio of 2.23 (95% CI: 1.50–3.32; P<0.0001) for 1-year mortality in multivariate logistic regression model (21). In contrast, Rio et al. did not discover an associated between CMV seropositivity and TRM in a prospective trial of adult patients receiving a RIC UCBT (26). Overall, the introduction of viral prophylaxis regimens and close monitoring of CMV reactivation has most likely downgraded its prognostic importance (50). Nevertheless, it is still imperative to test for CMV prior to transplantation, since it could identify patients who are at risk for reactivations.
Post-transplant lymphoproliferative disorder (PTLD) is a rare and feared complication, secondary to EBV reactivation, following allogeneic HSCT. Delayed immune reconstitution following UCBT puts recipients in excessive risk since patients should mount a T-cell response to avoid overt infection (51). EBV-PTLD risk in UCBT is dependent mainly on conditioning intensity and use of ATG. Brunstein et al. observed an incidence of EBV-related complications of 4.5% overall, 3.3% for myeloablative transplantations, and 7% for RIC transplantations (52). The leading complication was PTLD. Interestingly, the most striking difference was in a subset of patients treated with a RIC preparative regimen that included ATG versus those that did not (21% vs. 2%; P<0.01). When interpreting the results, it should be noted that the median age in the myeloablative group was 8 (range, 0.2–53) and 50 (range, 18–69) in patients receiving RIC (P<0.01). Furthermore, complications of EBV were rare, developing only in 15 of the 335 patients. Nevertheless, in a multivariate Cox regression on EBV complications, only RIC with ATG met statistical significance (HR of 15.4; 95% CI: 2.0–116.1, P<0.01, compared to myeloablative conditioning). Similar results were described by Dumas et al., who retrospectively studied EBV levels during the first three months after UCBT in 175 patients (53). The median age was 23 (range, 0.6–64), with 61% of patients being adults. Twenty-four patients presented with EBV reactivation of whom 4 had PTLD. More than 60% (15/24) developed reactivation during the first 100 days after transplant. In a univariate analysis, they noted that early EBV reactivation was associated with RIC in combination with ATG (P=0.03), previous history of auto-HSCT (P=0.01), and age ≥18 years (P=0.008) (53). These variables did not meet statistical significance in a multivariate analysis. Finally, in a retrospective analysis of 288 adults who received ATG, the 3-year cumulative incidence of PTLD related to EBV was 12.9% (95% CI: 3.2–22.5) and 2.6% (95% CI: 0.5–4.7) for patients receiving RIC and myeloablative conditioning, respectively (P<0.0001) (54). Overall, retrospective data suggest that a positive EBV serostatus pre-UCBT is not a major risk factor by itself, but is dependent on ATG administration. The risk for reactivation is probably related to delayed immune reconstitution following ATG (55). Post-UCBT EBV reactivation, on the other hand, could reflect the development of PTLD, which is a poor independent prognostic factor. Therefore, active EBV surveillance after transplantation is warranted.
Other markers of physiological reserve
Increasing life expectancy and better supportive care are among the many reasons of an increasing age of patients referred to a HSCT. Age per se does not necessarily reflect the candidates’ physiological reserve. Therefore, novel measures of fitness for transplantation are warranted. In a single-center prospective study, Muffly et al., have demonstrated the independent prognostic utility of a standardized geriatric assessment in 203 patients, aged 50 or more receiving an allogeneic HSCT (56). Twenty-eight (14%) patients had a cord blood donor. The comprehensive assessment included domains of function and disability, comorbidity, frailty, mental health, nutritional status, and systemic inflammation. Other promising prognostic markers of physiological reserve and inflammatory states are albumin, ferritin, and C-reactive protein (57,58). They have been tested in peripheral blood and bone marrow allogeneic HSCT, but have yet to be validated in the cord blood setting.
Predictive modeling in UCBT
Informed medical decisions are made on the basis of an estimated probability that a specific event will occur in the future in an individual. Such estimates of risk could be a major consideration in therapeutic decisions, risk stratification of patients in therapeutic intervention trials and analysis of retrospective data. Probability estimates are commonly based on combining information from multiple predictors observed or measured from an individual. Information from a single predictor is often insufficient to provide reliable estimates of prognostic probabilities or risks. Therefore, prediction models, also referred to as risk scores or prognostic models, integrating the prognostic weight of multiple predictors, are needed (59).
Prognostic models such as the EBMT risk score and the HCT-CI have been developed and validated in allogeneic HSCT (31,36). However, to date, there are no prediction models designed specifically for UCBT. Furthermore, only a few attempts have been made to apply allogeneic prediction models in the setting of cord blood. Wallet et al. showed that the EBMT score could stratify patients’ probability of OS and relapse (60). However, the overall predictive performance (i.e., c-statistic) and HRs were not reported, and there were some untested assumptions on EBMT risk score assignment (e.g., patients receiving a cord blood graft matched with at least 4/6 HLA alleles were scored as if they had a HLA matched sibling donor). The utility of the HCT-CI in UCBT has been reviewed above (Table 2). Data are suggestive that high scores (≥3) are associated with inferior OS and increased TRM. However, lower scores are not discriminatory, indicating a low predictive capacity. Development of prognostic models specifically for UCBT patients could potentially overcome the limitations described.
To develop a useful, robust, and accurate prediction model for OS or TRM following allogeneic HSCT, we recommend that investigators adhere to guidelines published as part of the Transparent Reporting of a multivariable prediction model for individual prognosis (TRIPOD) initiative (59,61). The TRIPOD statement provides a list of 22 items, deemed essential for transparent reporting of a prediction model study. An elaborate in-depth explanation of each item has been reviewed elsewhere (59). However, we would like to highlight several points, which could lead to improved predictive capacity.
Using repetitive computerized simulations in a large cohort of acute leukemia patients, we have previously shown that in traditional registry data only a few variables are predictive of mortality following allogeneic HSCT (62). It is likely that a similar phenomenon is present in cord blood registries. Therefore, it is our recommendation for permissive variable selection, integrating a wide variety of candidate predictors, similar to the ones suggested in Table 1. Another important feature in the development of a prediction model is the modeling technique. The vast majority of prognostic models in HSCT have been developed using Cox regression modelization. Indeed, Cox is a validated and robust method. However, it is limited in a number of important ways: assumptions must be made on data distribution, variable independence, and proportional hazards; models are parsimonious, requiring the preselection of variables and potentially losing predictive information; finally, conclusions are about data fitting the model and not a model fitting the data, forcing rigid assumptions about data behavior (63,64). Machine learning is an alternative modeling approach. It is a field in artificial intelligence, commonly applied in financial and technological settings, and is part of the mining approach for knowledge discovery. In machine learning, the underlying paradigm does not start with a pre-defined model; rather it lets the data create the model by detecting underlying patterns. Thus, this approach avoids pre-assumptions regarding model types and variable interactions and may provide new insight alongside the standard statistical methods (63,64).
Using machine learning to predict outcomes following UCBT is appealing, since there are dependencies in the data (e.g., number of cells in CBU and number of CBUs) and a likely violation of the proportional hazard assumption (i.e., the risk of a certain property remains stable over time) for some variables (e.g., conditioning regimen) (65). Our group has previously demonstrated the feasibility of the data mining approach in acute leukemia patients undergoing allogeneic HSCT. A machine learning based prediction model (the AL-EBMT score) for mortality 100 days post-transplant was constructed and validated on a large EBMT cohort (32). The Model identified predictive variables and several interactions. Discrimination was better than the EBMT score (c-statistic =0.701 versus 0.646, P value <0.001). Scores assigned were predictive of long-term outcomes as well. The AL-EBMT score has recently been validated in an independent cohort of acute leukemia patients from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) network (66).
Conclusions
Improving outcomes in the face of better supportive care have now made UCBT a feasible option for patients who would have been excluded only several years ago. Understanding the baseline physiological fitness of candidates could promote better patient selection. Moreover, it may serve as a driver for personalizing features of the transplantation itself, including conditioning and GVHD prophylaxis regimens. In the current review, we have summarized the literature on the prognostic role of patient-related features in UCBT. Furthermore, a road map for the development of comprehensive prediction models is proposed. Understanding the individual and global contribution of the various prognostic features involved in UCBT is essential to maximize benefit and minimize risk.
Acknowledgements
This study was supported by The Varda and Boaz Dotan Research Center in Hemato-Oncology affiliated with the CBRC of Tel Aviv University and The Shalvi Foundation for the Support of Medical Research.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ruggeri A. Alternative donors: cord blood for adults. Semin Hematol 2016;53:65-73. [Crossref] [PubMed]
- Querol S, Rubinstein P, Marsh SG, et al. Cord blood banking: 'providing cord blood banking for a nation'. Br J Haematol 2009;147:227-35. [Crossref] [PubMed]
- MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood 2009;113:2410-5. [Crossref] [PubMed]
- Rocha V, Wagner JE Jr, Sobocinski KA, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med 2000;342:1846-54. [Crossref] [PubMed]
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med 1997;337:373-81. [Crossref] [PubMed]
- Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med 1998;339:1565-77. [Crossref] [PubMed]
- Locatelli F, Rocha V, Chastang C, et al. Factors associated with outcome after cord blood transplantation in children with acute leukemia. Eurocord-Cord Blood Transplant Group. Blood 1999;93:3662-71. [PubMed]
- Komanduri KV, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood 2007;110:4543-51. [Crossref] [PubMed]
- Beck JC, Wagner JE, DeFor TE, et al. Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant 2010;16:215-22. [Crossref] [PubMed]
- Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood 2013;122:491-8. [Crossref] [PubMed]
- Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med 2001;344:1815-22. [Crossref] [PubMed]
- Cornetta K, Laughlin M, Carter S, et al. Umbilical cord blood transplantation in adults: results of the prospective Cord Blood Transplantation (COBLT). Biol Blood Marrow Transplant 2005;11:149-60. [Crossref] [PubMed]
- Barker JN, Rocha V, Scaradavou A. Optimizing unrelated donor cord blood transplantation. Biol Blood Marrow Transplant 2009;15:154-61. [Crossref] [PubMed]
- Barker JN, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood 2011;117:2332-9. [Crossref] [PubMed]
- Hamadani M, Craig M, Awan FT, et al. How we approach patient evaluation for hematopoietic stem cell transplantation. Bone Marrow Transplant 2010;45:1259-68. [Crossref] [PubMed]
- Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood 2013;121:2854-63. [Crossref] [PubMed]
- Milano F, Gooley T, Wood B, et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. N Engl J Med 2016;375:944-53. [Crossref] [PubMed]
- Eapen M, Klein JP, Ruggeri A, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood 2014;123:133-40. [Crossref] [PubMed]
- Hough R, Danby R, Russell N, et al. Recommendations for a standard UK approach to incorporating umbilical cord blood into clinical transplantation practice: an update on cord blood unit selection, donor selection algorithms and conditioning protocols. Br J Haematol 2016;172:360-70. [Crossref] [PubMed]
- Masuoka K, Uchida N, Ishiwata K, et al. What is the upper age limit for performing allo-SCT? Cord blood transplantation for an 82-year-old patient with AML. Bone Marrow Transplant 2011;46:619-20. [Crossref] [PubMed]
- Cohen YC, Scaradavou A, Stevens CE, et al. Factors affecting mortality following myeloablative cord blood transplantation in adults: a pooled analysis of three international registries. Bone Marrow Transplant 2011;46:70-6. [Crossref] [PubMed]
- Baron F, Labopin M, Ruggeri A, et al. Unrelated cord blood transplantation for adult patients with acute myeloid leukemia: higher incidence of acute graft-versus-host disease and lower survival in male patients transplanted with female unrelated cord blood--a report from Eurocord, the Acute Leukemia Working Party, and the Cord Blood Committee of the Cellular Therapy and Immunobiology Working Party of the European Group for Blood and Marrow Transplantation. J Hematol Oncol 2015;8:107. [Crossref] [PubMed]
- Tucunduva L, Ruggeri A, Sanz G, et al. Risk factors for outcomes after unrelated cord blood transplantation for adults with acute lymphoblastic leukemia: a report on behalf of Eurocord and the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2014;49:887-94. [Crossref] [PubMed]
- Ruggeri A, Sanz G, Bittencourt H, et al. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia 2014;28:779-86. [Crossref] [PubMed]
- Matsumura T, Kami M, Yamaguchi T, et al. Allogeneic cord blood transplantation for adult acute lymphoblastic leukemia: retrospective survey involving 256 patients in Japan. Leukemia 2012;26:1482-6. [Crossref] [PubMed]
- Rio B, Chevret S, Vigouroux S, et al. Decreased nonrelapse mortality after unrelated cord blood transplantation for acute myeloid leukemia using reduced-intensity conditioning: a prospective phase II multicenter trial. Biol Blood Marrow Transplant 2015;21:445-53. [Crossref] [PubMed]
- Robin M, Sanz GF, Ionescu I, et al. Unrelated cord blood transplantation in adults with myelodysplasia or secondary acute myeloblastic leukemia: a survey on behalf of Eurocord and CLWP of EBMT. Leukemia 2011;25:75-81. [Crossref] [PubMed]
- Rodrigues CA, Sanz G, Brunstein CG, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol 2009;27:256-63. [Crossref] [PubMed]
- Sorror M, Storer B, Sandmaier BM, et al. Hematopoietic cell transplantation-comorbidity index and Karnofsky performance status are independent predictors of morbidity and mortality after allogeneic nonmyeloablative hematopoietic cell transplantation. Cancer 2008;112:1992-2001. [Crossref] [PubMed]
- Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol 2014;32:3249-56. [Crossref] [PubMed]
- Gratwohl A, Stern M, Brand R, et al. Risk score for outcome after allogeneic hematopoietic stem cell transplantation: a retrospective analysis. Cancer 2009;115:4715-26. [Crossref] [PubMed]
- Shouval R, Labopin M, Bondi O, et al. Prediction of Allogeneic Hematopoietic Stem-Cell Transplantation Mortality 100 Days After Transplantation Using a Machine Learning Algorithm: A European Group for Blood and Marrow Transplantation Acute Leukemia Working Party Retrospective Data Mining Study. J Clin Oncol 2015;33:3144-51. [Crossref] [PubMed]
- Wagner JE Jr, Eapen M, Carter S, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med 2014;371:1685-94. [Crossref] [PubMed]
- Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood 2007;110:3064-70. [Crossref] [PubMed]
- Uchida N, Wake A, Takagi S, et al. Umbilical cord blood transplantation after reduced-intensity conditioning for elderly patients with hematologic diseases. Biol Blood Marrow Transplant 2008;14:583-90. [Crossref] [PubMed]
- Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005;106:2912-9. [Crossref] [PubMed]
- Elsawy M, Sorror ML. Up-to-date tools for risk assessment before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2016;51:1283-300. [Crossref] [PubMed]
- Sorror ML, Logan BR, Zhu X, et al. Prospective Validation of the Predictive Power of the Hematopoietic Cell Transplantation Comorbidity Index: A Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant 2015;21:1479-87. [Crossref] [PubMed]
- Majhail NS, Brunstein CG, Tomblyn M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant 2008;14:282-9. [Crossref] [PubMed]
- Smith AR, Majhail NS, MacMillan ML, et al. Hematopoietic cell transplantation comorbidity index predicts transplantation outcomes in pediatric patients. Blood 2011;117:2728-34. [Crossref] [PubMed]
- Nakaya A, Mori T, Tanaka M, et al. Does the hematopoietic cell transplantation specific comorbidity index (HCT-CI) predict transplantation outcomes? A prospective multicenter validation study of the Kanto Study Group for Cell Therapy. Biol Blood Marrow Transplant 2014;20:1553-9. [Crossref] [PubMed]
- DeFor TE, Majhail NS, Weisdorf DJ, et al. A modified comorbidity index for hematopoietic cell transplantation. Bone Marrow Transplant 2010;45:933-8. [Crossref] [PubMed]
- Abedin S, Peres E, Levine JE, et al. Double umbilical cord blood transplantation after novel myeloablative conditioning using a regimen of fludarabine, busulfan, and total lymphoid irradiation. Biol Blood Marrow Transplant 2014;20:2062-6. [Crossref] [PubMed]
- Gerds AT, Woo Ahn K, Hu ZH, et al. Outcomes after Umbilical Cord Blood Transplantation for Myelodysplastic Syndromes. Biol Blood Marrow Transplant 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Salit RB, Oliver DC, Delaney C, et al. Prognostic Value of the Hematopoietic Cell Transplantation Comorbidity Index for Patients Undergoing Reduced-Intensity Conditioning Cord Blood Transplantation. Biol Blood Marrow Transplant 2017;23:654-8. [Crossref] [PubMed]
- Fries BC, Riddell SR, Kim HW, et al. Cytomegalovirus disease before hematopoietic cell transplantation as a risk for complications after transplantation. Biol Blood Marrow Transplant 2005;11:136-48. [Crossref] [PubMed]
- Broers AE, van Der Holt R, van Esser JW, et al. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood 2000;95:2240-5. [PubMed]
- Jacobson CA, Turki AT, McDonough SM, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2012;18:565-74. [Crossref] [PubMed]
- Albano MS, Taylor P, Pass RF, et al. Umbilical cord blood transplantation and cytomegalovirus: Posttransplantation infection and donor screening. Blood 2006;108:4275-82. [Crossref] [PubMed]
- Milano F, Pergam SA, Xie H, et al. Intensive strategy to prevent CMV disease in seropositive umbilical cord blood transplant recipients. Blood 2011;118:5689-96. [Crossref] [PubMed]
- Rouce RH, Louis CU, Heslop HE. Epstein-Barr virus lymphoproliferative disease after hematopoietic stem cell transplant. Curr Opin Hematol 2014;21:476-81. [Crossref] [PubMed]
- Brunstein CG, Weisdorf DJ, DeFor T, et al. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood 2006;108:2874-80. [Crossref] [PubMed]
- Dumas PY, Ruggeri A, Robin M, et al. Incidence and risk factors of EBV reactivation after unrelated cord blood transplantation: a Eurocord and Societe Francaise de Greffe de Moelle-Therapie Cellulaire collaborative study. Bone Marrow Transplant 2013;48:253-6. [Crossref] [PubMed]
- Sanz J, Arango M, Senent L, et al. EBV-associated post-transplant lymphoproliferative disorder after umbilical cord blood transplantation in adults with hematological diseases. Bone Marrow Transplant 2014;49:397-402. [Crossref] [PubMed]
- Admiraal R, Lindemans CA, van Kesteren C, et al. Excellent T-cell reconstitution and survival depend on low ATG exposure after pediatric cord blood transplantation. Blood 2016;128:2734-41. [Crossref] [PubMed]
- Muffly LS, Kocherginsky M, Stock W, et al. Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica 2014;99:1373-9. [Crossref] [PubMed]
- Artz AS, Logan B, Zhu X, et al. The prognostic value of serum C-reactive protein, ferritin, and albumin prior to allogeneic transplantation for acute myeloid leukemia and myelodysplastic syndromes. Haematologica 2016;101:1426-33. [Crossref] [PubMed]
- Armand P, Kim HT, Cutler CS, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood 2007;109:4586-8. [Crossref] [PubMed]
- Moons KG, Altman DG, Reitsma JB, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1-73. [Crossref] [PubMed]
- Wallet HL, Sobh M, Robin M, et al. First application of the EBMT risk score in double umbilical cord blood transplantation for hematologic malignancies: significant impact on different outcomes. Exp Hematol 2014;42:161-2. [Crossref] [PubMed]
- Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015;350:g7594. [Crossref] [PubMed]
- Shouval R, Labopin M, Unger R, et al. Prediction of Hematopoietic Stem Cell Transplantation Related Mortality- Lessons Learned from the In-Silico Approach: A European Society for Blood and Marrow Transplantation Acute Leukemia Working Party Data Mining Study. PLoS One 2016;11:e0150637. [Crossref] [PubMed]
- Breiman L. Statistical modeling: The two cultures (with comments and a rejoinder by the author). Statistical science 2001;16:199-231. [Crossref]
- Shouval R, Bondi O, Mishan H, et al. Application of machine learning algorithms for clinical predictive modeling: a data-mining approach in SCT. Bone Marrow Transplant 2014;49:332-7. [Crossref] [PubMed]
- Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014;123:3664-71. [Crossref] [PubMed]
- Shouval R, Bonifazi F, Fein J, et al. Validation of the acute leukemia-EBMT score for prediction of mortality following allogeneic stem cell transplantation in a multi-center GITMO cohort. Am J Hematol 2017;92:429-34. [Crossref] [PubMed]
Cite this article as: Shouval R, Nagler A. From patient centered risk factors to comprehensive prognostic models: a suggested framework for outcome prediction in umbilical cord blood transplantation. Stem Cell Investig 2017;4:39.


