Immune reconstitution and outcomes after conditioning with anti-thymocyte-globulin in unrelated cord blood transplantation; the good, the bad, and the ugly
Introduction
Anti-thymocyte globulin (ATG) was implemented in the conditioning regimen in the early 1980’s to overcome the incidences of graft rejection and graft-versus-host-disease (GvHD) that were observed after transplantation with bone marrow (BM) or peripheral blood stem cells (PBSC) (1-4). It however became apparent that conditioning with ATG severely affects immune reconstitution (IR) (5-10). Especially, T-cell IR may be delayed or even absent in patients receiving ATG, which is associated with higher infection probability, relapse incidence, and subsequent lower survival chances. In the late 1980’s (1), unrelated umbilical cord blood (CB) was introduced as an alternative cell source for allogeneic hematopoietic (stem) cell transplantation (HCT) with important advantages. Unfortunately, in patients receiving unrelated umbilical cord blood transplantation (UCBT) an even more prominent ATG-dependent delay in T-cell IR was observed (10). However, in absence of ATG the IR (CD4+) potential of CB is as good as, or even better than, conventional stem cell sources (6,10-13), in pediatric cohorts. The ATG-induced delay in T-cell recovery thus limits the full potential of UCBT; either omitting ATG or using individualized dosing strategies may drastically improve UCBT outcome.
Nowadays, multiple formulations for ATG are available. ATG is either horse or rabbit derived, and can be produced using lymphocytes (Jurkat cells or whole thymus tissue). Several brands of ATG are on the market: Thymoglobulin® (rabbit derived, whole thymus, Sanofi-Genzyme), ATG-Fresenius® (rabbit derived, Jurkat cells, Fresenius/Neovi) and ATGAM® (equine, lymphocytes, Pfizer). In line with their differences in production, the different products differ in terms of pharmacokinetics (PK) and pharmacodynamics (PD); they require different dosing schemes. Thymoglobulin® is most frequently used in UCBT and, therefore, most knowledge on effects and mechanisms is available for this formulation. Throughout this review, ATG should be read as Thymoglobulin unless specified otherwise.
We will review the advantageous and adverse effects of ATG on outcomes and immune reconstitution after UCBT, and describe the mechanisms that are at play (Figure 1). Furthermore, we discuss what strategies can be used for future ATG conditioning; aimed at lowering GvHD and graft rejection risk, while optimally utilizing the potent T-cell recovery after UCBT.

The good; decreasing adverse alloreactive events after HCT
Why ATG was implemented in UCBT
One of the advantages of using CB as a cell source is that less stringent matching for human leukocyte antigens (HLA) is permitted, without increased probability of acute and chronic GvHD (aGvHD and cGvHD, respectively), compared to BM (14). This makes CB an interesting cell source, as for almost all pediatric patients a “well-matched” and adequately cell-dosed CB-graft can be selected. For adults and heavier weighted patients there can be more mismatches. Due to usually lower cell dose/kg, transplantation with two CB-units (double-cord) (15), or ex vivo expansion-strategies are available to obtain grafts containing enough cells for transplantation; such as nicotinamide (e.g., NiCord) (16), Delta1 Notch ligand (17), co-culture with mesenchymal-cells (e.g., Mesoblast) (18), aryl hydrocarbon receptor antagonist StemRegenin 1 (SR1, e.g., HSC835) (19), and the copper chelator tetraethylenepentamine (e.g., TEPA; StemEx) (20). However, even in a well-matched setting, graft rejection and GvHD incidences can be significant. To prevent adverse allogeneic reactions, ATG was implemented in the conditioning of UCBT, aiming for in vivo T-cell depletion of host cells (to prevent rejection) or donor cells (to prevent GvHD).
As ATG is a polyclonal antibody, it targets a large variety of antigens expressed on many immune cells, but also endothelial cells (21). ATG depletes immune cells either through direct apoptosis via the Fas/FasL pathway (22-24) or indirect cytotoxicity. The latter results from ATG binding to target cells and subsequent complement activation (complement-dependent-cytotoxicity; CDC), killing by natural killer (NK)-cells or granulocytes (antibody-dependent-cell-cytotoxicity; ADCC), or phagocytosis by monocytes and macrophages (antibody-dependent-cellular-phagocytosis; ADCP) (24,25). In addition to these cytotoxic properties, ATG can interfere with the function of B-cells, T-cells, natural killer-T-cells, and dendritic cells (DCs) (24). Notably, ATG exerts specific regulatory mechanisms by stimulating the development of regulatory T-cells (26-28), as well as the development of tolerogenic DCs (29). These provide other mechanisms by which ATG reduces GvHD risk. ATG also interferes with priming of allogeneic T-cells against patient-antigens, due to disruption of the T-cell - antigen-presenting-cell (APC) synapse (30). The latter reduces the chance of GvHD, as GvHD occurs when T-cells from an allogeneic graft are primed with patient-antigens by APCs.
Effects of ATG dosage on risk of graft rejection and GvHD
Most data on the effect of ATG on graft rejection and GvHD are available from BMT and PBSCT studies, mostly in adults. Early studies in BMT, PBSCT, and UCBT introduced conditioning with ATG at high dosages ranging from 7.5 to 20 mg/kg for Thymoglobulin or 30 to 60 mg/kg ATG-Fresenius. These studies not only reported decreased graft rejection, but also lower GvHD rates (31-34). Overall, conditioning with ATG appears to impact cGvHD more than aGvHD (31-34), but was associated with high rates of viral infection complications, and relapse (after myeloablative HCT). The severe toxicity observed in patients receiving these higher ATG dosages led to the use of lower doses of 4.5 to 10 mg/kg, and even 2.5 mg/kg, of Thymoglobulin, which still potently decreased both aGvHD and cGvHD (1-3,35). These studies indicate that all these regimens may be effective to a certain extent, but no clear survival advantages have been reported. A reason for this may be a highly variable exposure to ATG between individuals, due to variables influencing the pharmacokinetic profile. Until very recent, no PK and PD analyses were done to identify the optimal therapeutic window of ATG.
The effect of ATG exposure before and after transplantation on graft-versus-host disease risk
Our group recently performed in-depth PK and PD analyses of ATG in pediatric and adult patients in myeloablative and non-myeloablative conditioning using various cell sources. Interestingly, in the myeloablative setting we found that not the ATG exposure after transplantation, but the exposure before HCT predicted the probability of developing GvHD (10). ATG exposure after transplantation significantly impacted immune reconstitution and overall survival chances. The optimal area under the curve (AUC) before graft infusion was at least 40 active ATG-units × day/mL (10). Patients with this high exposure before either BMT or UCBT showed a lower probability of acute and chronic GvHD as well as lower rejection probability. In addition, Admiraal et al. evaluated the effect of ATG exposure after UCBT on the incidence of GvHD (11). This study showed that GvHD grade 2–4 probability is not impacted by ATG exposure after UCBT (11). These findings could imply that in vivo graft T-cell depletion by ATG exposure after transplantation might not be the most important factor in decreasing GvHD risk. We hypothesize that ATG exposure before transplantation induces suppression of host APCs (29,36), with possible depletion of these cells. As host APCs are shown to be most important to induce severe GvHD (37), suppression of these cells is pivotal to reduce GvHD risk. More research is, however, important to verify the effect of pre-HCT ATG exposure on lowering GvHD risk in other cohorts as well.
The bad and ugly; delaying T-cell recovery and ruining the unique properties of CB T-cells
ATG impacts immune reconstitution after HCT
Although conditioning with ATG resulted in decreased incidence of GvHD and graft rejection, overall survival chances were not improved after either BMT, PBSCT, or UCBT (7,31,38-42). This was due to an increased incidence of lethal infections, such as viral reactivations, which was pronounced in patients receiving high ATG doses (7,31,38,39,41). In addition, high ATG exposure after transplantation was related to increased relapse risk, in non-myeloablative HCT (43,44), but not in myeloablative HCT in adults (45-47). After myeloablative conditioning in pediatric BM or CB transplantation high exposure of ATG was associated with more relapse in AML patients (10). As adequate T-cell reconstitution is crucial to prevent viral reactivations (7,48,49) and relapse of disease (10,50-52), delayed T-cell recovery (5-10), caused by high ATG exposure after transplantation (7,10,11), thus highly influences survival chances after UCBT (9,10,53).
The in vivo depletion of graft T-cells by ATG exposure after UCBT is the most important reason for the delayed T-cell recovery. In the first months after transplantation, T-cell reconstitution is primarily dependent on homeostatic peripheral expansion (HPE) of graft T-cells. Depletion of graft T-cells by ATG thus severely hampers HPE. It is only after at least 6 months, or even years, until thymopoiesis contributes to T-cell recovery. The recovery of other immune subsets, such as NK-cells, B-cells, monocytes, granulocytes, and DCs, is less affected by ATG than T-cell recovery (5-10), despite that they also contain ATG epitopes (21). This may be due to the fact that recovery of these cells depends merely on direct BM differentiation and not on HPE like T-cells. As mentioned, when ATG is not part of the conditioning regimen, CB T-cell reconstitution is excellent, harboring unique anti-viral and anti-tumor properties. Usually within 1–2 months after UCBT hundreds of T-cells/µL are present (6,54). These T-cells also have proven to be functional as suggested by the lower incidence of viral reactivations compared to other cell sources (6,48,55), as well as lower incidence of relapse (50,56,57).
Residual ATG exposure after transplantation affects T-cell recovery after CB more than after BM transplantation
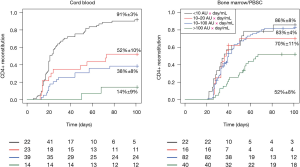
Compared to BMT or PBSCT, UCBT has been associated with a delayed and poor T-cell reconstitution (10,13,58-60). However, ATG was frequently used in these studies. We recently compared T-cell recovery in patients with different ATG exposure levels after UCBT and BMT/PBSCT (10). The probability of T-cell IR (>50×106 CD4+ T-cells/L blood within 100 days) was decreased even in the lowest ATG exposure group after UCBT, while only the highest ATG exposure group after BMT/PBSCT had poor T-cell IR (10). However, without ATG exposure, or with very low exposure, T-cell reconstitution after UCBT is at least as fast as BMT/PBSCT (5,6,59,60), or even faster (Figure 2). These findings indicate that delayed T-cell reconstitution reported after UCBT compared to BMT/PBSCT is explained by the fact that ATG more severely impacts T-cell recovery after UCBT. Our group is currently investigating what factors explain this higher effect on UCBT T-cell reconstitution by ATG exposure after transplantation.
The unique features of T-cells recovering after CB transplantation
Overcoming the detrimental effects of ATG on T-cell recovery is especially important to exploit the full potential of T-cell immunity after UCBT. Without ATG, the vastly naïve T-cells from the infused CB-graft show early expansion, with a rapid shift towards the central memory phenotype (6). Upon exposure to viruses, these CB-derived T-cells can be primed to become functional virus-specific T-cells as soon as 5–6 weeks after UCBT, able to resolve the viral infection/reactivation (6,55). This rapid ability to become antigen-specific may play a role in the low relapse rates observed in patients transplanted with CB compared to volunteer unrelated donors for a hematopoietic malignancy (50,56). Also in ex vivo models CB T-cells have a more powerful anti-leukemia function compared to PB T-cells (61). CB T-cells showed prompt induction of high IFNγ-producing CD8+ and CD4+ T-helper 1 T-cells, with rapid and functional tumor infiltration, which was significantly higher than PB T-cells (61). In addition, within two years after UCBT, a higher T-cell receptor (TCR)-diversity can be found compared to BMT (62). Early T-cell recovery with high TCR-diversity is related to higher survival chances (62,63). But to profit maximally from these unique properties of CB-derived T-cells, it is of utmost importance to optimize the ATG dosing aiming for either no or very low ATG after UCBT.
Effects of different ATG formulations on T-cell reconstitution and outcome
Different effects on immune reconstitution are observed with use of different ATG formulations. Most of these studies have been performed in BMT and PBSCT patients, but are likely to apply to the UCBT setting as well. T-cell recovery is significantly delayed in patients treated with rabbit-ATG (Thymoglobulin) (64) when compared with horse- (ATGAM). However, ATGAM did not show to be effective in preventing GvHD (65,66). This may be due to differences in avidity and affinity of the antibodies, or the amount of “active ATG”; the fraction of ATG that binds to human targets. This might explain why a higher concentration of ATG-Fresenius is needed to achieve cytotoxicity that is comparable to that of Thymoglobulin (67,68), and translates to a higher ATG-Fresenius dosage required for equal GvHD reduction (31-34). The European Blood and Marrow Transplant Group recommends 20 mg/kg/day (day-3 to day-1) ATG-Fresenius or 0.5 mg/kg Thymoglobulin (day-2) to 2 mg/kg (day-1 and day-1) in the myeloablative setting, or 2.5 mg/kg (days 2 and 1) Thymoglobulin in the reduced-intensity-conditioning setting, for GvHD prophylaxis in allogeneic HCT (69). However, with the current knowledge on PK, dosing based on mg/kg might still be too high for some of the patients, especially since exposure is relatively higher in heavier weighted patients (43,70).
ATG impacts T-cell recovery more severely in adults compared to children
Klein et al. investigated T-cell recovery in adults and children receiving UCBT after ATG conditioning (71). They investigated T-cell Receptor Excision Circles (sjTREC) in T-cells as well as TCR-diversity. They found a more diverse T-cell repertoire earlier in children (within 1–2 years after UCBT) than in adults (only after 3–4 years after UCBT). Overall, T-cell recovery was delayed in adults compared to T-cell recovery in children after conditioning with ATG (71,72). This fits the recent report that using weight-based dosing usually results in overdosing as clearance of ATG above the weight of 40 kg is only influenced by absolute lymphocyte count (43). Moreover, conditioning with ATG severely decreased sjTREC levels and TCR-diversity, which are both indicative for thymopoiesis, at least during the first 6–12 months after UCBT (6,71). Especially Thymoglobulin (rabbit-IgGs directed against human thymus cells) is found to damage the thymus and reduce thymopoiesis (73). Studies comparing TCR-diversity and sjTREC levels after UCBT with and without ATG in both children and adults are largely lacking to be able to assess to what extent these differences are impacted by age; e.g., thymus function. Furthermore, these observed disparities could well be explained by differences in exposure; when dosed on mg/kg, relatively higher ATG exposures are observed in patients with higher body weight (43,70). Thus, to optimally utilize the unique properties of CB T-cells, we need to harmonize and individualize ATG dosing (or omit ATG in certain patient groups).
Towards excellent T-cell reconstitution after UCBT; harboring the unique anti-viral and anti-tumor properties, while still preventing alloreactive adverse events
Omitting ATG from the conditioning regimen
Because of the detrimental effects of ATG on IR and outcome, some centers have recently chosen to omit ATG from the conditioning regimen prior to UCBT. This results in fast T-cell recovery (5,6,59,60), a lower risk of viral reactivations (48), and subsequent higher survival chances (49). CB-derived T-cells are known to mediate a more powerful anti-leukemic effect compared to adult T-cells (56,61,74,75). Omitting ATG has, therefore, shown to be feasible for patients with malignancies, especially since its detrimental effect on T-cell recovery was linked to higher relapse probability and lower survival chances (11,76). The reported aGvHD rates seem to be a bit higher, but manageable, while cGvHD rates remain low. The increased probability of aGvHD appears to mainly be a problem for patients transplanted with double UCBT (77,78). In non-immunodeficient benign disorders omitting ATG showed an increased incidence of graft-failure most likely due to insufficient depletion of host T-cells (5-7,59,60). Ideally, a conditioning regimen contains ATG aiming for very low exposure after transplantation to prevent the detrimental effect on IR, while maintaining a protective effect for graft-failure and aGvHD by having sufficiently high exposure before UCBT (10,11).
Low-dose ATG or ATG earlier in conditioning
In BMT and PBSCT, low-dose ATG (2.5 mg/kg Thymoglobulin, or 35 mg/kg ATG-Fresenius) decreased GvHD risk (35,79), with minimal impact on immune reconstitution (72). Nevertheless, IR was still severely delayed compared to no ATG (5,6,59,60). Giving ATG already at day-9 before transplantation also lowers exposure after transplantation, while improving IR (7,10,11). These strategies are still based on dosing on body weight only, which has been shown to result in over- or under exposure in a significant number of patients (43,70). In addition, even very low ATG exposure after transplantation has shown to highly affect IR after UCBT (10,11). It was recently found that clearance of ATG was mainly influenced by body-weight (<40 kg) and absolute lymphocyte count (43). Therefore, dosing ATG at a fixed mg/kg dose for all weights, and not taking the absolute lymphocyte count into account, will still result in high variability in ATG exposures between patients, subsequently causing unpredictable T-cell recovery.
Individualized ATG dosing
The most reliable method to obtain optimal ATG exposure is through individualized dosing and therapeutic drug monitoring (TDM). In individualized dosing, the dose and start day of ATG is chosen based on factors impacting ATG PK, such as body weight, lymphocyte count, and HCT cell source (70). Furthermore, TDM could be advantageous to monitor ATG exposure, and target for optimal exposure in high-risk patients. The advantage of individualized dosing is that the pharmacokinetic model targets for low exposure after HCT; providing optimal T-cell IR, while maintaining high exposure before HCT; protecting against GvHD and graft rejection (10,11). A prospective clinical trial (Dutch trial register NTR4960) is currently underway to investigate this approach in pediatric UCBT and BMT.
Lowering GvHD further potentiates T-cell reconstitution
Important to note is that a strategy to limit GvHD with low ATG exposure after UCBT may further improve T-cell reconstitution, as GvHD itself also is a major factor affecting T-cell recovery (80). This is partly due to the need for immunosuppressive therapy (steroids) to treat GvHD, and in part to the detrimental effect of the GvHD reaction on thymus cells and thymopoiesis (80-82). Therapeutic drug monitoring for immunosuppressive drugs, such as tacrolimus (83), cyclosporine (84), and mycophenolate mofetil (85), would be helpful to target to the appropriate exposure to limit GvHD with a less profound effect on T-cell recovery. Future research is needed to find the balance between thymic damage from GvHD and immunosuppression to treat GvHD.
Concluding remarks and future perspectives for the use of ATG in UCBT
Generally, ATG has shown to be successful in preventing graft rejection and GvHD in allogeneic HCT using different cell sources. Exposure of ATG after HCT, however, has a tremendous negative impact on survival chances due to delayed T-cell recovery. Notably, when ATG exposure after transplantation is low, T-cell recovery and function might even be superior after UCBT compared to BMT and PBSCT. Recent studies have given us clues to better understand why UCBT has historically been related to delayed T-cell IR; ATG exposure after UCBT affects T-cell IR to a higher extent than after BMT and PBSCT. Additional findings indicate that adequate ATG levels before transplantation are important to target alloreactive adverse effects, while low or absent ATG exposure after transplantation is crucial for early T-cell recovery.
Future research should aim for a more predictable T-cell recovery after UCBT, which is pivotal to achieve optimal outcomes. Individualized ATG dosing and TDM have the highest potential to obtain optimal ATG exposure that does not affect IR, while still preventing GvHD, in each individual patient. Although this review only focused on the effect of ATG conditioning on T-cell recovery after UCBT, more strategies to tackle delayed T-cell recovery exist (86). CB T-cells have unique properties that are currently under-utilized by conditioning with ATG. These unique anti-viral/anti-tumor properties may be better utilized by implementing non-inherited maternal antigens (NIMA)-matching (87,88), inherited paternal antigens (IPA)-mismatching (87,89,90), and optimal Predicted Indirectly ReCognizable HLA Epitopes (PIRCHE) (91). By individualizing ATG in the conditioning, UCBT provides a unique platform for HCT, with optimal and potent T-cell immunity associated with low GvHD and high anti-virus and GvL properties.
Acknowledgements
Funding: This work was supported by Foundation Children Cancerfree (KiKa) project number 142 and by the Netherlands Organization for Health Research and Development (ZonMW) grant number 40-41500-98-11044.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Crocchiolo R, Esterni B, Castagna L, et al. Two days of antithymocyte globulin are associated with a reduced incidence of acute and chronic graft-versus-host disease in reduced-intensity conditioning transplantation for hematologic diseases. Cancer 2013;119:986-92. [Crossref] [PubMed]
- Remberger M, Ringdén O, Hägglund H, et al. A high antithymocyte globulin dose increases the risk of relapse after reduced intensity conditioning HSCT with unrelated donors. Clin Transplant 2013;27:E368-74. [Crossref] [PubMed]
- Pascal L, Mohty M, Ruggeri A, Tucunduva L, et al. Impact of rabbit ATG-containing myeloablative conditioning regimens on the outcome of patients undergoing unrelated single-unit cord blood transplantation for hematological malignancies. Bone Marrow Transplant 2015;50:45-50. [Crossref] [PubMed]
- Theurich S, Fischmann H, Shimabukuro-Vornhagen A, et al. Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults. Cochrane Database Syst Rev 2012;12:CD009159. [PubMed]
- Booth C, Veys P. T cell depletion in paediatric stem cell transplantation. Clin Exp Immunol 2013;172:139-47. [Crossref] [PubMed]
- Chiesa R, Gilmour K, Qasim W, et al. Omission of in vivo T-cell depletion promotes rapid expansion of naïve CD4+ cord blood lymphocytes and restores adaptive immunity within 2 months after unrelated cord blood transplant. Br J Haematol 2012;156:656-66. [Crossref] [PubMed]
- Lindemans CA, Chiesa R, Amrolia PJ, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood 2014;123:126-32. [Crossref] [PubMed]
- Bosch M, Dhadda M, Hoegh-Petersen M, et al. Immune reconstitution after anti-thymocyte globulin-conditioned hematopoietic cell transplantation. Cytotherapy 2012;14:1258-75. [Crossref] [PubMed]
- Willemsen L, Jol-van der Zijde CM, Admiraal R, et al. Impact of Serotherapy on Immune Reconstitution and Survival Outcomes After Stem Cell Transplantations in Children: Thymoglobulin Versus Alemtuzumab. Biol Blood Marrow Transplant 2015;21:473-82. [Crossref] [PubMed]
- Admiraal R, van Kesteren C, Jol-van der Zijde CM, et al. Association between anti-thymocyte globulin exposure and CD4+ immune reconstitution in paediatric haemopoietic cell transplantation: a multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol 2015;2:e194-203. [Crossref] [PubMed]
- Admiraal R, Lindemans CA, van Kesteren C, et al. Excellent T-cell reconstitution and survival provided ATG exposure is low or absent after pediatric cord blood transplantation. Blood 2016;128:2734-41. [Crossref] [PubMed]
- Moretta A, Maccario R, Fagioli F, et al. Analysis of immune reconstitution in children undergoing cord blood transplantation. Exp Hematol 2001;29:371-9. [Crossref] [PubMed]
- Oshrine BR, Li Y, Teachey DT, et al. Immunologic recovery in children after alternative donor allogeneic transplantation for hematologic malignancies:comparison of recipients of partially T cell-depleted peripheral blood stem cells and umbilical cord blood. Biol Blood Marrow Transplant 2013;19:1581-9. [Crossref] [PubMed]
- Horowitz MM. High-resolution typing for unrelated donor transplantation:How far do we go? Best Pract Res Clin Haematol 2009;22:537-41. [PubMed]
- Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 2005;105:1343-7. [Crossref] [PubMed]
- Horwitz ME, Chao NJ, Rizzieri DA, et al. Clinical medicine Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest 2014;124:3121-8. [Crossref] [PubMed]
- Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med 2010;16:232-6. [Crossref] [PubMed]
- de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med 2012;367:2305-15. [Crossref] [PubMed]
- Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 2010;329:1345-8. [Crossref] [PubMed]
- Stiff PJ, Montesinos P, Peled T, et al. StemEx®(Copper Chelation Based) Ex Vivo Expanded Umbilical Cord Blood Stem Cell Transplantation (UCBT) Accelerates Engraftment and Improves 100 Day Survival In Myeloablated Patients Compared To a Registry Cohort Undergoing Double Unit UCBT: Results Of a Multicenter Study Of 101 Patients With Hematologic Malignancies. Blood 2013;122:295.
- Popow I, Leitner J, Grabmeier-Pfistershammer K, et al. A Comprehensive and Quantitative Analysis of the Major Specificities in Rabbit Antithymocyte Globulin Preparations. Am J Transplant 2013;13:3103-13. [Crossref] [PubMed]
- Dubey S, Nityanand S. Involvement of Fas and TNF pathways in the induction of apoptosis of T cells by antithymocyte globulin. Ann Hematol 2003;82:496-9. [Crossref] [PubMed]
- Neff KS, Richards SM, Williams JM, et al. Murine Antithymocyte Globulin T-Cell Depletion Is Mediated Predominantly by Macrophages, but the Fas/FasL Pathway Selectively Targets Regulatory T Cells. Transplantation 2011;92:523-8. [Crossref] [PubMed]
- Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia 2007;21:1387-94. [Crossref] [PubMed]
- Zand MS, Vo T, Pellegrin T, et al. Apoptosis and complement-mediated lysis of myeloma cells by polyclonal rabbit antithymocyte globulin. Blood 2006;107:2895-903. [Crossref] [PubMed]
- Feng X, Kajigaya S, Solomou EE, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+CD25highFOXP3+ regulatory T cells in vitro. Blood 2008;111:3675-83. [Crossref] [PubMed]
- Shimony O, Nagler A, Gellman YN, et al. Anti-T Lymphocyte Globulin (ATG) Induces Generation of Regulatory T Cells, at Least Part of Them Express Activated CD44. J Clin Immunol 2012;32:173-88. [Crossref] [PubMed]
- LaCorcia G, Swistak M, Lawendowski C, et al. Polyclonal Rabbit Antithymocyte Globulin Exhibits Consistent Immunosuppressive Capabilities Beyond Cell Depletion. Transplantation 2009;87:966-74. [Crossref] [PubMed]
- Gillet-Hladky S, de Carvalho CM, Bernaud J, et al. Rabbit Antithymocyte Globulin Inhibits Monocyte-Derived Dendritic Cells Maturation In Vitro and Polarizes Monocyte-Derived Dendritic Cells Towards Tolerogenic Dendritic Cells Expressing Indoleamine 2,3-dioxygenase. Transplantation 2006;82:965-74. [Crossref] [PubMed]
- Haidinger M, Geyeregger R, Poglitsch M, et al. Antithymocyte Globulin Impairs T-Cell/Antigen-Presenting Cell Interaction:Disruption of Immunological Synapse and Conjugate Formation. Transplantation 2007;84:117-21. [Crossref] [PubMed]
- Bacigalupo A, Lamparelli T, Bruzzi P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001;98:2942-7. [Crossref] [PubMed]
- MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood 2009;113:2410-5. [Crossref] [PubMed]
- Finke J, Bertz H, Schmoor C, et al. Allogeneic bone marrow transplantation from unrelated donors using in vivo anti-T-cell globulin. Br J Haematol 2000;111:303-13. [Crossref] [PubMed]
- Wolschke C, Zabelina T, Ayuk F, et al. Effective prevention of GVHD using in vivo T-cell depletion with anti-lymphocyte globulin in HLA-identical or -mismatched sibling peripheral blood stem cell transplantation. Bone Marrow Transplant 2014;49:126-30. [Crossref] [PubMed]
- Bryant A, Mallick R, Huebsch LB, et al. Low-Dose Anti-Thymocyte Globulin for Graft-Versus-Host-Disease Prophylaxis in Matched Unrelated Allogeneic Hematopoietic Stem Cell Transplant. Blood 2016;128:5782.
- Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science 1999;285:412-5. [Crossref] [PubMed]
- Koyama M, Kuns RD, Olver SD, et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat Med 2011;18:135-42. [Crossref] [PubMed]
- Bacigalupo A, Lamparelli T, Barisione G, et al. Thymoglobulin Prevents Chronic Graft-versus-Host Disease, Chronic Lung Dysfunction, and Late Transplant-Related Mortality: Long-Term Follow-Up of a Randomized Trial in Patients Undergoing Unrelated Donor Transplantation. Biol Blood Marrow Transplant 2006;12:560-5. [Crossref] [PubMed]
- Wang Y, Fu HX, Liu DH, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: a randomized trial. Bone Marrow Transplant 2014;49:426-33. [Crossref] [PubMed]
- Ruggeri A, Sanz G, Bittencourt H, et al. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia 2014;28:779-86. [Crossref] [PubMed]
- Socie G, Schmoor C, Bethge WA, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood 2011;117:6375-82. [Crossref] [PubMed]
- Kumar A, Mhaskar AR, Reljic T, et al. Antithymocyte globulin for acute-graft-versus-host-disease prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation: a systematic review. Leukemia 2012;26:582-8. [Crossref] [PubMed]
- Admiraal R, Nierkens S, de Witte MA, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: a multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol 2017;4:e183-91. [Crossref] [PubMed]
- Hannon M, Beguin Y, Ehx G, et al. Immune Recovery after Allogeneic Hematopoietic Stem Cell Transplantation Following Flu-TBI versus TLI-ATG Conditioning. Clin Cancer Res 2015;21:3131-9. [Crossref] [PubMed]
- Walker I, Panzarella T, Couban S, et al. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol 2016;17:164-73. [Crossref] [PubMed]
- Kröger N, Solano C, Wolschke C, et al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N Engl J Med 2016;374:43-53. [Crossref] [PubMed]
- Baron F, Labopin M, Blaise D, et al. Impact of in vivo T-cell depletion on outcome of AML patients in first CR given peripheral blood stem cells and reduced-intensity conditioning allo-SCT from a HLA-identical sibling donor: a report from the Acute Leukemia Working Party of the European group for Blood and Marrow Transplantation. Bone Marrow Transplant 2014;49:389-96. [Crossref] [PubMed]
- Hiwarkar P, Gaspar HB, Gilmour K, et al. Impact of viral reactivations in the era of pre-emptive antiviral drug therapy following allogeneic haematopoietic SCT in paediatric recipients. Bone Marrow Transplant 2013;48:803-8. [Crossref] [PubMed]
- Admiraal R, de Koning C, Bierings MB, et al. Viral reactivations and associated outcomes in context of immune reconstitution after pediatric hematopoieitc cell transplantation. J Allergy Clin Immunol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Parkman R, Cohen G, Carter SL, et al. Successful immune reconstitution decreases leukemic relapse and improves survival in recipients of unrelated cord blood transplantation. Biol Blood Marrow Transplant 2006;12:919-27. [Crossref] [PubMed]
- Ishaqi MK, Afzal S, Dupuis A, et al. Early lymphocyte recovery post-allogeneic hematopoietic stem cell transplantation is associated with significant graft-versus-leukemia effect without increase in graft-versus-host disease in pediatric acute lymphoblastic leukemia. Bone Marrow Transplant 2008;41:245-52. [Crossref] [PubMed]
- Admiraal R, Chiesa R, Bierings M, et al. Early CD4+ Immune Reconstitution Predicts Probability of Relapse in Pediatric AML after Unrelated Cord Blood Transplantation: Importance of Preventing in Vivo T-Cell Depletion Using Thymoglobulin®. Biol Blood Marrow Transplant 2015;21:S206. [Crossref]
- Bartelink IH, Belitser SV, Knibbe CA, et al. Immune Reconstitution Kinetics as an Early Predictor for Mortality using Various Hematopoietic Stem Cell Sources in Children. Biol Blood Marrow Transplant 2013;19:305-13. [Crossref] [PubMed]
- Flinsenberg TWH, Spel L, Jansen M, et al. Cognate CD4 T-Cell Licensing of Dendritic Cells Heralds Anti-Cytomegalovirus CD8 T-Cell Immunity after Human Allogeneic Umbilical Cord Blood Transplantation. J Virol 2015;89:1058-69. [Crossref] [PubMed]
- Cohen G, Carter SL, Weinberg KI, et al. Antigen-specific T-lymphocyte function after cord blood transplantation. Biol Blood Marrow Transplant 2006;12:1335-42. [Crossref] [PubMed]
- Milano F, Gooley T, Wood B, et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. N Engl J Med 2016;375:944-53. [Crossref] [PubMed]
- Verneris MR, Brunstein CG, Barker J, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood 2009;114:4293-9. [Crossref] [PubMed]
- Szabolcs P, Niedzwiecki D. Immune reconstitution after unrelated cord blood transplantation. Cytotherapy 2007;9:111-22. [Crossref] [PubMed]
- Jacobson CA, Turki A, McDonough S, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2012;18:565-74. [Crossref] [PubMed]
- Kanda J, Chiou LW, Szabolcs P, et al. Immune recovery in adult patients after myeloablative dual umbilical cord blood, matched sibling, and matched unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012;18:1664-76. [Crossref] [PubMed]
- Hiwarkar P, Qasim W, Ricciardelli I, et al. Cord blood T cells mediate enhanced anti-tumor effects compared with adult peripheral blood T cells. Blood 2015;126:2882-91. [Crossref] [PubMed]
- Talvensaari K, Clave E, Douay C, et al. A broad T-cell repertoire diversity and an efficient thymic function indicate a favorable long-term immune reconstitution after cord blood stem cell transplantation. Blood 2002;99:1458-64. [Crossref] [PubMed]
- Emerson RO, Nikiforow S, Milano F, et al. TCR Repertoire Diversity Assessed with Immunosequencing Is Associated with Patient Mortality Following Cord Blood Transplant. Blood 2014;124:1262.
- Scheinberg P, Nunez O, Weinstein B, et al. Horse versus Rabbit Antithymocyte Globulin in Acquired Aplastic Anemia. N Engl J Med 2011;365:430-8. [Crossref] [PubMed]
- Hagen P, Wagner JE, DeFor TE, et al. The effect of equine antithymocyte globulin on the outcomes of reduced intensity conditioning for AML. Bone Marrow Transplant 2014;49:1498-504. [Crossref] [PubMed]
- Champlin RE, Perez WS, Passweg JR, et al. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood 2007;109:4582-5. [Crossref] [PubMed]
- Popow I, Leitner J, Majdic O, J., et al. Assessment of Batch to Batch Variation in Polyclonal Antithymocyte Globulin Preparations. Transplantation 2012;93:32-40. [Crossref] [PubMed]
- Naujokat C, Berges C, Fuchs D, et al. Antithymocyte Globulins Suppress Dendritic Cell Function by Multiple Mechanisms. Transplantation 2007;83:485-97. [Crossref] [PubMed]
- Baron F, Mohty M, Blaise D, et al. Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: a review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica 2017;102:224-34. [Crossref] [PubMed]
- Admiraal R, van Kesteren C, Jol-van der Zijde CM, et al. Population Pharmacokinetic Modeling of Thymoglobulin® in Children Receiving Allogeneic-Hematopoietic Cell Transplantation: Towards Improved Survival Through Individualized Dosing. Clin Pharmacokinet 2015;54:435-46. [Crossref] [PubMed]
- Klein AK, Patel DD, Gooding ME, et al. T-Cell recovery in adults and children following umbilical cord blood transplantation. Biol Blood Marrow Transplant 2001;7:454-66. [Crossref] [PubMed]
- Castillo N, García-Cadenas I, Barba P, et al. Early and Long-Term Impaired T Lymphocyte Immune Reconstitution after Cord Blood Transplantation with Antithymocyte Globulin. Biol Blood Marrow Transplant 2017;23:491-7. [Crossref] [PubMed]
- Na IK, Wittenbecher F, Dziubianau M, et al. Rabbit antithymocyte globulin (thymoglobulin) impairs the thymic output of both conventional and regulatory CD4+ T cells after allogeneic hematopoietic stem cell transplantation in adult patients. Haematologica 2013;98:23-30. [Crossref] [PubMed]
- Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia:a comparison study. Lancet 2007;369:1947-54. [Crossref] [PubMed]
- Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: Results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011;118:282-8. [Crossref] [PubMed]
- Soiffer RJ, LeRademacher J, Ho V, et al. Impact of immune modulation with anti-T-cell antibodies on the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Blood 2011;117:6963-70. [Crossref] [PubMed]
- Ponce DM, Gonzales A, Lubin M, et al. Graft-versus-Host Disease after Double-Unit Cord Blood Transplantation Has Unique Features and an Association with Engrafting Unit-to-Recipient HLA Match. Biol Blood Marrow Transpl 2011;17:1316-26. [Crossref]
- Wagner JE, Eapen M, Carter S, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med 2014;371:1685-94. [Crossref] [PubMed]
- Binkert L, Medinger M, Halter JP, et al. Lower dose anti-thymocyte globulin for GvHD prophylaxis results in improved survival after allogeneic stem cell transplantation. Bone Marrow Transplant 2015;50:1331-6. [Crossref] [PubMed]
- Clave E, Busson M, Douay C, et al. Acute graft-versus-host disease transiently impairs thymic output in young patients after allogeneic hematopoietic stem cell transplantation. Blood 2009;113:6477-84. [Crossref] [PubMed]
- Holländer GA, Widmer B, Burakoff SJ. Loss of normal thymic repertoire selection and persistence of autoreactive T cells in graft vs host disease. J Immunol 1994;152:1609-17. [PubMed]
- Hauri-Hohl MM, Keller MP, Gill J, et al. Donor T-cell alloreactivity against host thymic epithelium limits T-cell development after bone marrow transplantation. Blood 2007;109:4080-8. [Crossref] [PubMed]
- Wallin JE, Friberg LE, Fasth A, et al. Population Pharmacokinetics of Tacrolimus in Pediatric Hematopoietic Stem Cell Transplant Recipients: New Initial Dosage Suggestions and a Model-Based Dosage Adjustment Tool. Ther Drug Monit 2009;31:457-66. [Crossref] [PubMed]
- Dupuis LL, Seto W, Teuffel O, et al. Prediction of Area under the Cyclosporine Concentration Versus Time Curve in Children Undergoing Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 2013;19:418-23. [Crossref] [PubMed]
- Bhatia M, Militano O, Jin Z, et al. An Age-Dependent Pharmacokinetic Study of Intravenous and Oral Mycophenolate Mofetil in Combination with Tacrolimus for GVHD Prophylaxis in Pediatric Allogeneic Stem Cell Transplantation Recipients. Biol Blood Marrow Transplant 2010;16:333-43. [Crossref] [PubMed]
- de Koning C, Nierkens S, Boelens JJ. Strategies before, during, and after hematopoietic cell transplantation to improve T-cell immune reconstitution. Blood 2016;128:2607-15. [Crossref] [PubMed]
- van Rood JJ, Scaradavou A, Stevens CE. Indirect evidence that maternal microchimerism in cord blood mediates a graft-versus-leukemia effect in cord blood transplantation. Proc Natl Acad Sci U S A 2012;109:2509-14. [Crossref] [PubMed]
- Mold JE, Michaëlsson J, Burt TD, et al. Maternal Alloantigens Promote the Development of Tolerogenic Fetal Regulatory T Cells in Utero. Science 2008;322:1562-5. [Crossref] [PubMed]
- Burlingham WJ, Nelson JL. Microchimerism in cord blood:mother as anticancer drug. Proc Natl Acad Sci U S A 2012;109:2190-1. [Crossref] [PubMed]
- Milano F. Fetal maternal immunity and antileukemia activity in cord-blood transplant recipients. Bone Marrow Transplant 2013;48:321-2. [Crossref] [PubMed]
- Thus KA, de Hoop TA, de Weger RA, et al. Predicted Indirectly ReCognizable HLA Epitopes Class I Promote Antileukemia Responses after Cord Blood Transplantation: Indications for a Potential Novel Donor Selection Tool. Biol Blood Marrow Transplant 2016;22:170-3. [Crossref] [PubMed]
Cite this article as: de Koning C, Admiraal R, Nierkens S, Boelens JJ. Immune reconstitution and outcomes after conditioning with anti-thymocyte-globulin in unrelated cord blood transplantation; the good, the bad, and the ugly. Stem Cell Investig 2017;4:38.


