Hypomethylating agents after allogeneic blood stem cell transplantation
Introduction
For many patients with acute myeloid leukemia (AML) allogeneic blood stem cell transplantation (allo-SCT) offers the highest potential for long-term survival, while for patients with myelodysplastic syndromes (MDS) allo-SCT is the only curative treatment option (1). During the last decades, many advances have been made to reduce non-relapse mortality, such as improvements in donor selection, immunosuppression and supportive care. Furthermore, the introduction of reduced toxicity conditioning as well as the use of alternative donor sources have improved outcome and also broadened the access for more, in particular older patients to this treatment option (2).
Despite these advances concerning the pre- and direct transplant phase, relapse still represents the main cause of treatment failure and is associated with a poor prognosis. Treatment options in this challenging situation are limited and have generally consisted of palliative care, low-dose or intensive chemotherapy as well as cellular therapies such as donor lymphocyte infusions (DLI) and second transplantation in selected cases. However, many patients can either not tolerate intensive therapies or are refractory to those conventional interventions (3). Thus, there is a need for novel treatment approaches, which on the one side exert a direct antileukemic effect and ideally direct the donor immune system towards an enhanced graft-versus-leukemia (GvL) reaction. On the other hand, such a therapy should have an acceptable toxicity profile and prevent severe graft-versus-host disease (GvHD).
The two hypomethylating agents (HMA) azacitidine (Aza) and decitabine (DAC) might provide these properties. Both are licensed for the treatment of older patients with AML and/or MDS not eligible for intensive therapies due to their balance between good efficacy and moderate toxicity (4-7). Based on these considerations others and we have tested these substances in the post-transplant period either alone or in combination with DLI.
This review aims to summarize the current knowledge about the use of Aza and DAC to prevent or to treat relapse of AML and MDS after allo-SCT. In addition, we will also give an overview about ongoing research and clinical studies to investigate the use of these two HMA after transplant.
HMA for the treatment of relapse
Aza for the treatment of relapse
Considering the poor outcome after conventional salvage therapies our group treated the first patient with early relapse of an AML evolved from MDS after allo-SCT with Aza and DLI in 2007 (8). Although this was rather due to the lack of realistic treatment alternatives than a decision based on a pathophysiological rationale, we were successful and this woman achieved a complete remission (CR) following this combined pharmacological and cell-based approach. Following this first case, a few small retrospective studies reported on the use of Aza as salvage therapy for relapse of myeloid malignancies after allo-SCT (9-11). These data built the rationale for the first prospective multicenter trial (AZARELA, Eudra-CT 2007-004860-37) (12). In this study, Aza had to be the first intervention for the treatment of relapse and DLI were envisaged in all patients (Figure 1).
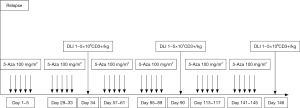
The majority of patients (92%) included in this trial suffered from AML (15 de novo AML, 5 secondary following MDS) and 2 patients had MDS and MDS-MPS, respectively. All patients had hematologic relapse and received a median of 3 courses Aza (range, 1–8 courses) and 22 patients (73%) finally received DLI.
Following this treatment, we observed an encouraging overall response rate of 47%. Seven patients (23%) achieved CR, 2 patients (7%) partial remission (PR), and 5 patients (17%) had stable disease (SD). Of the 7 patients who achieved CR, 5 patients continued in CR for a median of 777 days (range, 461−890 days) without any additional treatment. Interestingly, this approach was especially effective in patients with high-risk cytogenetics as 6 of the 7 patients who achieved CR had a complex karyotype. In contrast, the rate and severity of GvHD as well as toxicities following the treatment with Aza and DLI were rather mild and compared well, if not better with other treatment options. Together with the experience from the early retrospective reports this prospective trial demonstrated that the combination of Aza and DLI was safe and effective in patients with AML or MDS who relapse after allo-SCT.
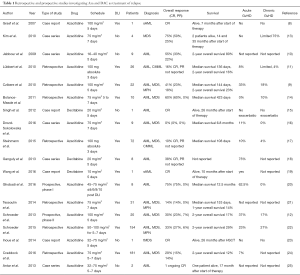
Overall, as indicated in Table 1, a total of 215 patients with AML, MDS and other related myeloid malignancies had been reported in the literature until 2015 (8-21,23,25). Treatment schedules and dosages of Aza varied between these reports. Furthermore, Aza was the first treatment of relapse and combined with DLI in some patients, while other patients had previously received other salvage therapies or did not receive DLI. Probably, due to this heterogeneity CR rates and overall survival (OS, not given in details in a relevant number of many studies) ranged from 14% to 60% and from 12% to 80%, respectively.
Full table
In addition, this heterogeneity and limited number of patients in the retrospective studies but even in the prospective trial did not enable to identify predictive factors for response and long-term survival. This prompted us to perform a retrospective analysis of 154 patients with relapse of AML or MDS after allo-HSCT who were treated with Aza and were scheduled for DLI at 12 German transplantation centers (22). The majority of these patients suffered from hematologic relapse (88%), whereas 19 patients (12%) had molecular relapse. In 143 patients (93%), Aza was the first therapy for relapse, while 11 patients (7%) had received antileukemic treatment before Aza therapy. Patients received a median of 4 courses Aza (range, 4−14 courses) and DLI were administered to 105 patients (68%).
The size of this patients group and the quality of data provided by the participating centers allowed us to identify patients who may benefit most from the combination of Aza and DLI. In multivariate analyses, in particular patients with MDS and those with a molecular relapse had a significantly higher probability to achieve CR following Aza and DLI. This also applied to OS, which was predicted to be significantly longer in patients with MDS as well as in those with a low disease burden (molecular relapse or bone marrow blast count <13%) at the time of relapse.
In general, some of the findings were recently confirmed by results from a retrospective EBMT analysis investigating a similar-sized patient group (n=181) (24). Here, Craddock and colleagues identified the diagnosis of MDS instead of AML and transplantation in remission as predictors for response. For OS disease burden defined by the bone marrow blast count at the time of relapse (cut-off 20%) and a longer interval between allo-SCT and relapse (cut-offs 6 and 12 months) turned out to be predictive in multivariate analysis. Based on these variables the authors proposed a so-called AZA Relapse Prognostic Score (ARPS). Although this score could clearly divide their cohort in 3 prognostically different subgroups, this score has not been validated in an independent cohort yet. Another drawback of this study is that only patients with haematological, but not molecular relapse were included. Given the opportunity of continuously optimized molecular methods to monitor minimal residual disease (MRD) to direct MRD-triggered interventions after transplant, a cut-off of 20% BM blasts cast the practicability of this score for real life into doubt.
Despite these controversies, these two large analyses together with the prospective and retrospective studies have univocally shown that the combination of HMA and DLI is of therapeutic value for patients relapsing after allo-SCT, in particular for those with MDS or AML with low disease burden.
DAC for the treatment of relapse
In contrast to Aza, the evidence regarding the use of DAC, the second HMA approved in Europe for the treatment of AML, as salvage therapy for relapse after allo-SCT is limited covering a total of 9 patients so far (Table 1). In these three case series 5 patients achieved CR and no excess of toxicity was reported (15,18,26). Although these reports suggest that DAC might also have some efficacy in patients with myeloid malignancies relapsing after allo-SCT, they do not provide sufficient information on survival and response rates due to the limited number of patients. Results from prospective trials investigating DAC as salvage therapy for relapse after allo-SCT have not been published so far and, to the best of our knowledge, will also not be available in the near future.
HMA for the prevention of relapse
Maintenance vs. consolidation therapy with HMA after allo-SCT
Even though HMA can induce long-term remissions in a relevant number of patients with relapse after allo-SCT, it is rather better to avoid than to treat relapse. One potential approach to reduce the risk of AML or MDS relapse following allo-SCT could be prophylactic therapy. Prophylactic approaches can be separated into maintenance or consolidation therapies. While the former means a continuous therapy until disease progression or intolerability, the latter represents a therapy phase defined by a limited time interval and/or number of treatment cycles. Independent from this rather academic distinction, such prophylactic treatment may be either able to directly eliminate MRD or to control disease activity until the donor immune system is sufficiently reconstituted to mediate the desired GvL effect.
In accordance with this definition, no maintenance trials but 5 prospective single-arm studies investigating Aza (n=3) or DAC (n=2) as consolidation therapy for patients with AML or MDS after allo-SCT have been published (27-31). The major aims of these early-phase studies covering a total of 130 patients were to demonstrate feasibility and to find an appropriate dose for future trials based on the balance between tolerability and efficacy. As indicated in Table 2 consolidation therapy was planned to start within the first 2 to 3 months after transplant in order to cope with the fact that most relapses occur within the first year after transplant. However, treatment onset was delayed due to toxicity reasons in most trials. In addition, in the trials of Craddock et al. and de Lima et al., 27% and 50% of enrolled patients dropped out prior the first administration of Aza as a consequence of toxicity, patient wish or relapse (27,28). Major adverse events related to the study drugs were hepatotoxicity and infections. Although the doses of Aza and DAC were significantly lower than the approved dosages in the non-transplant setting only a minority of patients could receive the complete number of envisaged treatment cycles. Overall, this indicates, that the study population represents a selected group of patients and highlights the potential risks of post-transplant cytotoxic therapy. Given the limited number of patients and the lack of a control arm, a definitive ranking of outcome results in-terms of relapse incidence, survival and GvHD is impossible so far. However, with regard to the risk of GvHD, there was a hint in the study of de Lima et al. that post-transplant therapy with Aza might be associated with a lower probability to develop chronic GvHD.

Full table
Finally, the Aza dose identified in the study of Lima et al. provided the basis for an ongoing randomized phase III trial investigating Aza for relapse prevention after allo-SCT in patients with myeloid malignancies (NCT00887068) (28). In this trial, 246 patients will be included and receive Aza or placebo for a period of 1 year after transplant. Estimated primary completion date is April 2018 and results from this trial will hopefully elucidate the impact of Aza-based consolidation on relapse risk and GvHD.
Pre-emptive therapy with HMA
Although consolidation therapy may be an important concept, the following concerns must be raised. Generally, allo-SCT is a potentially curative therapy and many patients with high-risk myeloid malignancies have a chance to achieve long-term cure without post-transplant cytotoxic therapy. Therefore, it is obvious that for some patients HMA given after transplant may represent over-treatment associated with potentially detrimental side effects in “already cured” patients. This can include cytopenias and infections but also eventually secondary malignancies in the long-term.
To deal with this, MRD-triggered pre-emptive therapy including DLI instead of treatment in remission may be a better strategy. In our large retrospective analysis, we recently found that treatment at the time of molecular instead of haematological relapse was associated with a higher likelihood of remission and survival (22). This idea has also been addressed by Platzbecker and colleagues in a prospective trial, where pre-emptive Aza therapy was guided by donor chimerism in circulating CD34+ cells. Twenty patients with decreasing CD34+ donor chimerism (<80%) who still were in haematological remission received treatment with up to 4 cycles of Aza. Although an improvement of chimerism (>80%) was observed in 50% (10 of 20) of patients, haematological relapse occurred in the majority of patients and continuous remission was only achieved in 3 (30%) of the responders (32). This was probably related to the limited number of Aza cycles, but in particular to the fact that DLI were not part of the protocol. The same group has employed this approach in patients with NPM1-mutated AML including 3 of them with MRD after allo-SCT highlighting this concept to guide post-transplant interventions (33).
The recent discovery of several distinctive gene mutations in patients with myeloid malignancies by genomic high-throughput techniques together with technical advances regarding PCR-based methods will enable a stringent MRD monitoring for the majority of patients. This will help to employ and optimize MRD-based pre-emptive therapies with HMA and other compounds in the close future.
Mode of action
The underlying mechanisms, by which HMA mediate relevant anti-leukemic efficacy accompanied by a relatively low incidence and severity of GvHD, have been addressed in animal models but also in translational analyses of human samples. Besides a primarily cytotoxic effect lower doses of HMA have shown to upregulate several antigens including HLA epitopes, cancer testis antigens and minor histocompatibility molecules on leukemic cells in vitro and in vivo (34-37). This re-expression of epigenetically silenced genes by HMA is thought to render malignant cells more immunogenic toward T-cell killing. Along with this, several groups have shown effects of HMA on T cell mediated antitumor activity by increasing tumor-specific CD8 T cell responses, for example against cancer testis antigens (38). Furthermore, treatment with DNA-demethylating agents can induce cell surface expression of formerly unexpressed killer Ig-like receptors (KIRs) in natural killer cells and may hereby contribute to a HMA-mediated GvL effect (39) .
The reproducible observation of relatively infrequent and mostly mild GvHD also supported the idea that HMA might offer immunoregulatory properties. In this context, two groups demonstrated that HMA convert conventional T cells to Tregs and hereby prevent GVHD after allogeneic transplant or DLI in mice with no effect on GVL. HMA after allo-SCT and DLI reduce GvHD in these animals by a direct T cell suppression and by conversion of alloreactive donor T cells into Tregs (CD4+, CD25+, FOXP3+) through enhancement of FOXP3 expression (40,41). Expansion of Tregs has also been observed in humans who received Aza either as maintenance or salvage therapy for relapsed AML and MDS (42,43). Taken together, these results suggest that Aza might target different immunological pathways and may hereby separate GvHD and GvL to a certain extent. Still, many of the underlying mechanisms need to be deciphered to gain a better understanding of the molecular and immunologic events associated with the use of HMA after allo-SCT.
Open questions
Overall, HMA and in particular Aza have proven to be a valuable treatment alternative for relapse. Still, most of the evidence is based on retrospective reports, but not on prospective randomized trial covering a large number of patients. As a consequence, several questions regarding the practical use of HMA as salvage therapy after transplant have not been answered sufficiently yet. Here, we comment on some of these issues based on the current knowledge:
What is the optimal dose and number of cycles of HMA for treatment of relapse?
With regard to this question no definitive answer can be given. As indicated in Table 1 different schedules with daily dosages ranging from 16 to 100 mg/m2 for 3 to 7 days have been used. Responses have been observed with all dosages and there is no clear relationship between dosage and response. One could assume that a potentially higher anti-leukemic effect mediated by a higher dose might be relevant for patients with high leukemic burden or rapid relapse kinetics but should be balanced against potential side effects, in particular cytopenias and cytopenia related complications.
We currently start with the approved Aza dosage of 75 mg/m2 for 7 days in accordance to the protocol of our prospective trial (12) and adapt dosages during the following cycles in case of hematotoxicity.
Median time and number of cycles to response also varied between the reports. This suggests, that whenever feasible at least 4 cycles should be administered before a definitive evaluation of response can be made. In addition, also duration of treatment is not defined by any evidence from the literature, whether to administer a definitive number of cycle or to continue until progression or intolerability as recommended in the non-transplant setting. Again, we try to follow the scheme of our prospective trial and administer 6 to 8 cycles of Aza if feasible. In addition, we aim to infuse DLI after every second Aza cycle with increasing T cell dosages until GvHD occurs. Based on our personal experience, we administer at least 1 cycle of Aza after the last DLI, since we have observed some cases of severe GvHD in cases where DLI was the last intervention.
Aza or DAC—which one to choose for relapse after allo-SCT?
No randomized trial has addressed this question so far. As described above, both HMA can to induce remissions in patients relapsing after allo-SCT. However, currently the literature definitively covers more patients treated with Aza than with DAC in this situation, including 2 prospective trials. Furthermore, in Europe DAC is only licensed for patients with AML but not MDS. For this reason, we generally use Aza in this setting and only consider DAC in patients with contraindications against Aza or in case of Aza failure. Furthermore, DAC might also an alternative for AML patients with high blast counts or rapid disease kinetics at relapse.
Are DLI and/or second transplant needed in addition to HMA?
Again, this relevant question, whether a combination with donor cells is required for response and long-term survival, cannot be answered on the basis of prospective randomized trials. The results of the studies published so far were heterogeneous regarding the use of DLI and second transplant (Table 1).
In the recent retrospective EBMT analysis, the administration of DLI had no impact on either the probability of achieving a major response or on 2-year OS. However, in this analysis only 38% of patients (n=69) received DLI, and only those 39 of whom received DLI within two months of commencing AZA salvage and in the absence of a clinical response were included in multivariate analysis (24). Besides this methodological limitation, these results are in strict contrast to results from another retrospective EBMT analysis. Here, Schmid and colleagues clearly demonstrated, that reinduction of CR by pharmacological compounds including Aza alone is not sufficient for long-term survival, but donor-cell-based consolidation is required (44). The results from our retrospective analysis are in further support of this idea, as in 78% of patients CR was obtained after the first DLI, suggesting a pronounced cell-induced immune reaction. Remissions induced by Aza and DLI in our analysis were ongoing for a median period of 20 months in 66% of patients and lasted for a median of 13 months in those who finally relapsed again (22). In contrast, in the study of Platzbecker et al., even though it was given pre-emptively, Aza alone could prevent hematologic relapse only in a minority of patients, probably related to the fact that DLI were not part of the protocol (32).
Therefore, to us it seems reasonable to follow the general principle that relapse after allo-SCT needs treatment with a cellular approach either alone or in combination with cytotoxic therapy.
Prophylactic or preemptive therapy with HMA?
As discussed above, prophylactic treatment might represent over-treatment associated with relevant side effects in a relevant proportion of patients. In the absence of randomized trials demonstrating a benefit of a prophylactic approach with HMA so far, no recommendation for its use can be made and patients should be treated in clinical trials.
In the future, to tailor post-transplant treatment a special attention has to be paid to patient selection based on relapse risk. One approach for a better risk stratification could be the knowledge about the adverse prognosis of karyotype alterations, gene mutations and their combination. For example, some studies have shown that TP53 mutations indicate a dismal prognosis for MDS patients after allo-SCT and may be able to subdivide patients with complex karyotypes with regard to their prognosis (45,46). The goal is to identify a patient population with an extraordinary high relapse risk for further studies to test innovative post-transplant strategies applied in remission. In patients with an intermediate risk for relapse after allo-SCT over-treatment should be avoided. Therefore, MRD-triggered, pre-emptive therapy including DLI instead of treatment in remission may be a better strategy as recent studies have shown that it can achieve excellent outcomes.
Potential combination partners
In the non-transplant setting several compounds such as HDAC inhibitors, tyrosine kinase inhibitors (TKI) or the immunomodulator lenalidomide have been tested in combination with HMA. Based on initial favourable results from these trials (47) some combinations are currently also under investigation for treatment of relapse after allo-SCT. For example, there are currently 2 trials investigating the potentially additive effect of lenalidomide (VIOLA trial and NCT02472691). In the ongoing trials others and we hope to exploit a potential stimulation of the donor immune system by lenalidomide in order to enhance the Aza-mediated GvL effect.
Another interesting class of drugs are TKI, which have inhibitory effects on internal tandem duplications (ITD) in the gene encoding for the Fms-like tyrosine-3 (FLT3) kinase receptor. One of these candidates is sorafenib, a multikinase inhibitor with activity against FLT3 kinase. Sorafenib with or without DLI has demonstrated antileukemic activity in this situation and may induce complete molecular remissions in some patients (48,49). Furthermore, given the promising results of a recent phase-II trial combining Sorafenib and Aza in relapsed or refractory FLT3−ITD mutated AML (50), this combination may also be worth testing after transplant in patients with FLT3−ITD+ AML.
Finally, immune checkpoint blockade is also entering the field of haematological malignancies. There are early preliminary signals from in-vitro and in-vivo systems suggesting that a combination of HMA and PD1-blocking agents may have a pathophysiological rationale in AML and MDS (51,52). Clinical trials investigating such approaches have just started in the non-transplant setting and, if successful, might be expanded to the field of relapse after allo-SCT.
Conclusions
HMA and in particular Aza have proven to be a valuable treatment for MDS and AML patients relapsing after allo-SCT. A better understanding of the underlying mechanisms and identification of target patient populations will hopefully help to further optimize this approach. In addition, the arrival of new pharmacological compounds together with the upcoming improvements of specialized cellular products and antibodies may also help to further improve prognosis of relapse after allo-SCT.
Acknowledgements
None.
Footnote
Conflicts of Interest: T Schroeder had a consulting role for Celgene Corporation, Germany and received financial travel support and lecture fees from Celgene Corporation, Germany. C Rautenberg received financial travel support from Celgene Corporation, Germany. R Haas has nothing to declare. G Kobbe received financial travel support, research funding and lecture fees from Celgene Corporation, Germany.
References
- Sureda A, Bader P, Cesaro S, et al. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant 2015;50:1037-56. [Crossref] [PubMed]
- Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med 2010;363:2091-101. [Crossref] [PubMed]
- de Lima M, Porter DL, Battiwalla M, et al. Proceedings from the National Cancer Institute's Second International Workshop on the Biology, Prevention, and Treatment of Relapse After Hematopoietic Stem Cell Transplantation: part III. Prevention and treatment of relapse after allogeneic transplantation. Biol Blood Marrow Transplant 2014;20:4-13. [Crossref] [PubMed]
- Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015;126:291-9. [Crossref] [PubMed]
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009;10:223-32. [Crossref] [PubMed]
- Fenaux P, Mufti GJ, Hellström-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol 2010;28:562-9. [Crossref] [PubMed]
- Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012;30:2670-7. [Crossref] [PubMed]
- Graef T, Kuendgen A, Fenk R, et al. Successful treatment of relapsed AML after allogeneic stem cell transplantation with azacitidine. Leuk Res 2007;31:257-9. [Crossref] [PubMed]
- Czibere A, Bruns I, Kröger N, et al. 5-Azacytidine for the treatment of patients with acute myeloid leukemia or myelodysplastic syndrome who relapse after allo-SCT: a retrospective analysis. Bone Marrow Transplant 2010;45:872-6. [Crossref] [PubMed]
- Jabbour E, Giralt S, Kantarjian H, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer 2009;115:1899-905. [Crossref] [PubMed]
- Lübbert M, Bertz H, Wäsch R, et al. Efficacy of a 3-day, low-dose treatment with 5-azacytidine followed by donor lymphocyte infusions in older patients with acute myeloid leukemia or chronic myelomonocytic leukemia relapsed after allografting. Bone Marrow Transplant 2010;45:627-32. [Crossref] [PubMed]
- Schroeder T, Czibere A, Platzbecker U, et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia 2013;27:1229-35. [Crossref] [PubMed]
- Kim SY, Cho SG, Cho BS, et al. Azacytidine treatment after discontinuation of immunosuppressants in patients with myelodysplastic syndrome and relapse after allo-SCT at a single center. Bone Marrow Transplant 2010;45:1375-6. [Crossref] [PubMed]
- Bolaños-Meade J, Smith BD, Gore SD, et al. 5-azacytidine as salvage treatment in relapsed myeloid tumors after allogeneic bone marrow transplantation. Biol Blood Marrow Transplant 2011;17:754-8. [Crossref] [PubMed]
- Singh SN, Cao Q, Gojo I, et al. Durable complete remission after single agent decitabine in AML relapsing in extramedullary sites after allo-SCT. Bone Marrow Transplant 2012;47:1008-9. [Crossref] [PubMed]
- Drozd-Sokołowska J, Gil L, Waszczuk-Gajda A, et al. Azacitidine Use After Allogeneic Stem Cell Transplantation-Results From the Polish Adult Leukemia Group. Transplant Proc 2016;48:1802-5. [Crossref] [PubMed]
- Steinmann J, Bertz H, Wäsch R, et al. 5-Azacytidine and DLI can induce long-term remissions in AML patients relapsed after allograft. Bone Marrow Transplant 2015;50:690-5. [Crossref] [PubMed]
- Ganguly S, Amin M, Divine C, et al. Decitabine in patients with relapsed acute myeloid leukemia (AML) after allogeneic stem cell transplantation (allo-SCT). Ann Hematol. 2013;92:549-50. [Crossref] [PubMed]
- Wang B, Jin X, Wang Q, et al. Decitabine+ CAG +DLI in relapsed acute myeloid leukemia after allogeneic stem cell transplantation. J BUON 2016;21:280-1. [PubMed]
- Ghobadi A, Choi J, Fiala MA, et al. Phase I study of azacitidine following donor lymphocyte infusion for relapsed acute myeloid leukemia post allogeneic stem cell transplantation. Leuk Res 2016;49:1-6. [Crossref] [PubMed]
- Tessoulin B, Delaunay J, Chevallier P, et al. Azacitidine salvage therapy for relapse of myeloid malignancies following allogeneic hematopoietic SCT. Bone Marrow Transplant 2014;49:567-71. [Crossref] [PubMed]
- Schroeder T, Rachlis E, Bug G, et al. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions--a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant 2015;21:653-60. [Crossref] [PubMed]
- Inoue A, Kawakami C, Takitani K, et al. Azacitidine in the treatment of pediatric therapy-related myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation. J Pediatr Hematol Oncol 2014;36:e322-4. [Crossref] [PubMed]
- Craddock C, Labopin M, Robin M, et al. Clinical activity of azacitidine in patients who relapse after allogeneic stem cell transplantation for acute myeloid leukemia. Haematologica 2016;101:879-83. [Crossref] [PubMed]
- Antar A, Otrock ZK, Kharfan-Dabaja M, et al. Azacitidine in the treatment of extramedullary relapse of AML after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2013;48:994-5. [Crossref] [PubMed]
- Phillips CL, Davies SM, McMasters R, et al. Low dose decitabine in very high risk relapsed or refractory acute myeloid leukaemia in children and young adults. Br J Haematol 2013;161:406-10. [Crossref] [PubMed]
- Craddock C, Jilani N, Siddique S, et al. Tolerability and Clinical Activity of Post-Transplantation Azacitidine in Patients Allografted for Acute Myeloid Leukemia Treated on the RICAZA Trial. Biol Blood Marrow Transplant 2016;22:385-90. [Crossref] [PubMed]
- de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer 2010;116:5420-31. [Crossref] [PubMed]
- Han S, Kim YJ, Lee J, et al. Model-based adaptive phase I trial design of post-transplant decitabine maintenance in myelodysplastic syndrome. J Hematol Oncol 2015;8:118. [Crossref] [PubMed]
- Oshikawa G, Kakihana K, Saito M, et al. Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk acute myeloid leukaemia. Br J Haematol 2015;169:756-9. [Crossref] [PubMed]
- Pusic I, Choi J, Fiala MA, et al. Maintenance Therapy with Decitabine after Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transplant 2015;21:1761-9. [Crossref] [PubMed]
- Platzbecker U, Wermke M, Radke J, et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia 2012;26:381-9. [Crossref] [PubMed]
- Sockel K, Wermke M, Radke J, et al. Minimal residual disease-directed preemptive treatment with azacitidine in patients with NPM1-mutant acute myeloid leukemia and molecular relapse. Haematologica 2011;96:1568-70. [Crossref] [PubMed]
- Almstedt M, Blagitko-Dorfs N, Duque-Afonso J, et al. The DNA demethylating agent 5-aza-2'-deoxycytidine induces expression of NY-ESO-1 and other cancer/testis antigens in myeloid leukemia cells. Leuk Res 2010;34:899-905. [Crossref] [PubMed]
- Atanackovic D, Luetkens T, Kloth B, et al. Cancer-testis antigen expression and its epigenetic modulation in acute myeloid leukemia. Am J Hematol 2011;86:918-22. [Crossref] [PubMed]
- Hambach L, Ling KW, Pool J, et al. Hypomethylating drugs convert HA-1-negative solid tumors into targets for stem cell-based immunotherapy. Blood 2009;113:2715-22. [Crossref] [PubMed]
- Pinto A, Maio M, Attadia V, et al. Modulation of HLA-DR antigens expression in human myeloid leukaemia cells by cytarabine and 5-aza-2'-deoxycytidine. Lancet 1984;2:867-8. [Crossref] [PubMed]
- Goodyear O, Agathanggelou A, Novitzky-Basso I, et al. Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood 2010;116:1908-18. [Crossref] [PubMed]
- Santourlidis S, Trompeter HI, Weinhold S, et al. Crucial role of DNA methylation in determination of clonally distributed killer cell Ig-like receptor expression patterns in NK cells. J Immunol 2002;169:4253-61. [Crossref] [PubMed]
- Choi J, Ritchey J, Prior JL, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood 2010;116:129-39. [Crossref] [PubMed]
- Sánchez-Abarca LI, Gutierrez-Cosio S, Santamaría C, et al. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood 2010;115:107-21. [Crossref] [PubMed]
- Goodyear OC, Dennis M, Jilani NY, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia (AML). Blood 2012;119:3361-9. [Crossref] [PubMed]
- Schroeder T, Fröbel J, Cadeddu RP, et al. Salvage therapy with azacitidine increases regulatory T cells in peripheral blood of patients with AML or MDS and early relapse after allogeneic blood stem cell transplantation. Leukemia 2013;27:1910-3. [Crossref] [PubMed]
- Schmid C, Labopin M, Nagler A, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood 2012;119:1599-606. [Crossref] [PubMed]
- Bejar R, Stevenson KE, Caughey B, et al. Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J Clin Oncol 2014;32:2691-8. [Crossref] [PubMed]
- Della Porta MG, Gallì A, Bacigalupo A, et al. Clinical Effects of Driver Somatic Mutations on the Outcomes of Patients With Myelodysplastic Syndromes Treated With Allogeneic Hematopoietic Stem-Cell Transplantation. J Clin Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Sekeres MA, Tiu RV, Komrokji R, et al. Phase 2 study of the lenalidomide and azacitidine combination in patients with higher-risk myelodysplastic syndromes. Blood 2012;120:4945-51. [Crossref] [PubMed]
- Metzelder SK, Schroeder T, Finck A, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia 2012;26:2353-9. [Crossref] [PubMed]
- Schroeder T, Zohren F, Saure C, et al. Sorafenib treatment in 13 patients with acute myeloid leukemia and activating FLT3 mutations in combination with chemotherapy or as monotherapy. Acta Haematol 2010;124:153-9. [Crossref] [PubMed]
- Ravandi F, Alattar ML, Grunwald MR, et al. Phase 2 study of azacytidine plus sorafenib in patients with acute myeloid leukemia and FLT-3 internal tandem duplication mutation. Blood 2013;121:4655-62. [Crossref] [PubMed]
- Ørskov AD, Treppendahl MB, Skovbo A, et al. Hypomethylation and up-regulation of PD-1 in T cells by azacytidine in MDS/AML patients: A rationale for combined targeting of PD-1 and DNA methylation. Oncotarget 2015;6:9612-26. [Crossref] [PubMed]
- Yang H, Bueso-Ramos C, DiNardo C, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 2014;28:1280-8. [Crossref] [PubMed]
Cite this article as: Schroeder T, Rautenberg C, Haas R, Kobbe G. Hypomethylating agents after allogeneic blood stem cell transplantation. Stem Cell Investig 2016;3:84.


