Enrichment of skin-derived neural precursor cells from dermal cell populations by altering culture conditions
Introduction
Skin-derived neural precursor cells (skin-NPCs) were isolated and characterized as skin-derived precursors (SKPs) in 2001 (1). SKPs are a distinct population of skin stem cells which exhibit properties of neural crest (NC) precursors. These NC-derived precursors migrated into the skin during embryogenesis and maintained their multipotency until adulthood like their NC ancestors (2). Similar to their potential developmental origin, these skin-NPCs can generate both neural and mesodermal progeny and differentiate into the separate subpopulation of cells expressing neuronal, glial, smooth muscle, adipocyte, and osteoblast markers (3). On the basis of the hypothesis that skin could contain a neural precursor differentiating into the Merkel cells, SKPs were isolated as neurospheres, the most common culture form of neural stem cells, for the first time (1). In general, two different culture forms can be used for isolation and expansion of NPCs: non-adherent spherical clusters of cells or neurospheres and adherent monolayer cultures (4). Many studies have stated that culturing NPCs as neurospheres has some disadvantages such as heterogeneity of cells due to different accessibility of cells to the growth factor and difficult and uncertain monitoring of cells under the inverted microscope (5-7). In recent years, Babu and the colleague created a protocol to isolate and propagate neural stem cells by adherent monolayer cultures. They demonstrated that this method could meet the problems associated with culturing cells as neurospheres and that it represents a more homogeneous undifferentiated population of precursor cells (4,8). Moreover, adherent monolayer culture has been introduced as an efficient method to isolate neural stem cells of different parts of rodent brains (8). In the present study, a novel and simple protocol was designed consisted of serum-supplemented and serum-free media to increase dermal neurogenic cell population by monolayer adherent culture. Finally, the protein markers and the differentiation potential were examined in isolated and cultured cells. The results showed that these isolated cells express nestin, fibronectin and vimentin, markers of SKPs, and have the capacity to differentiate into the neuronal, glial, adipogenic, osteogenic and myogenic lineages after being isolated and expanded by the same culture form.
Methods
Cell isolation
Skin-NPCs were isolated and propagated by a protocol previously described by Babu et al. with some modification (4,8). It should be mentioned that all experiments were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee and with the guidelines for care and use of experimental animals required by Ahvaz Jundishapur University of Medical Sciences (AJUMS). Skin from adult rat (male Albino Wistar, 8 weeks and older) was dissected from the dorsum of the animal and cut into 1×1 cm2 pieces. Skin pieces were incubated in thermolysin (Sigma, NY, USA) overnight at 4 °C. The epidermis was manually removed, and the dermis was minced and incubated in collagenase type 1 (Sigma, NY, USA) for 50–60 min at 37 °C. The digested tissues were mechanically dissociated and filtered through a 40 µm cell strainer (Falcon, BD Biosciences, San Diego, CA). Dissociated cells were pelleted and cultured as follows.
In the first step, dissociated cells were plated in DMEM-F12 (3:1; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA) until confluence. Afterwards, cells were cultured in DMEM-F12 containing 2% B27, 20 ng/mL EGF and 40 ng/mL FGF2 (Peprotech, Rocky Hill, NJ). Medium was changed every 72 h until it reached confluence. Cells were cultured in 25-cm tissue culture flasks (Falcon, BD Biosciences, San Diego, CA) in a 37 °C, 5% CO2 tissue-culture incubator. Finally, differentiation potential and protein markers of isolated cells were evaluated in cultured cells.
As control, dissociated dermal cells were plated in DMEM-F12 (3:1; Invitrogen, Carlsbad, CA) supplemented with 10% FBS (Invitrogen) until the end of the experiments.
Immunofluorescence
After 14 days of cultivation, cells of both test and control groups at passage 3 were rinsed with PBS, fixed by 4% paraformaldehyde (Sigma, NY, USA) for 20 min and permeabilized with 0.5% Triton X 100 (Merck, NJ, USA) for 10 min. Thereafter, cells were blocked by 3% Bovine serum albumin for 2 h (Sigma, NY, USA) and incubated with the following primary antibodies for 2 h at 4 °C: monoclonal anti-nestin, monoclonal anti-fibronectin, monoclonal anti-vimentin, monoclonal anti-βIII tubulin, monoclonal anti-GFAP, and monoclonal anti-myosin (fast skeletal, 1:100) (Sigma, NY, USA), Then, cells were rinsed with PBS three times and incubated with goat anti-mouse FITC conjugated secondary antibody (1:150) (Sigma, NY, USA) for 2 h at room temperature in darkness. Finally, cells were examined under the Zeiss fluorescence microscope. It should be mentioned that the corresponding negative controls were set using secondary antibodies without adding primary antibodies. Therefore, any observed fluorescence resulted from the nonspecific binding of secondary antibody to the sample.
To obtain an estimate of the percentage of cells expressing a given marker protein, at least five fields were photographed for any given experiment, and the number of positive cells was determined relative to the total number of DAPI-labeled nuclei.
Differentiation potential assay
To confirm the multipotential capacity of isolated cells, these cells were cultured in different differentiation medium and differentiated down the neuronal, glial, adipogenic, osteogenic and myogenic lineages.
For neuronal differentiation, cells were cultured in DMEM-F12 (3:1) supplemented with 50 ng/mL NGF (Peprotech, Rocky Hill, NJ) and 10% FBS for 7 days.
For Schwann cell differentiation, cells were cultured in DMEM-F12 (3:1) supplemented with 10% FBS for 7 days, thereafter cultured in medium supplemented with 4 µM forskolin (Sigma, NY, USA).
To induce adipocyte differentiation, Skin-NPCs were cultured in DMEM-F12 (3:1) supplemented with 25% FBS for 14 days.
Osteogenic differentiation was promoted by culturing the subconfluent cells in DMEM containing 50 µM ascorbate-2 phosphate (Sigma, NY, USA), 10 mM β-glycerophosphate (Sigma, NY, USA) and 0.1 µM dexamethasone for 15 days. Mineralized colonies were visualized by alizarin red S (Sigma, NY, USA).
To promote myogenic differentiation, high density cells were cultured in proliferation medium on the plates coated with type I collagen for 10 days. Myotube-like structures were observed under by the inverted microscopy and Myogenin as a marker showing the myogenic commitment of differentiating SKPs was characterized by immunocytochemistry.
Statistical analysis
All data are presented as mean ± SEM from at least three independent experiments performed. Statistical analysis was performed using SPSS software (version 21.0; SPSS Inc., Chicago, IL). One-way ANOVA was used to analyze the mean values statistically. Significance was set at P<0.05.
Results
Morphological assessment
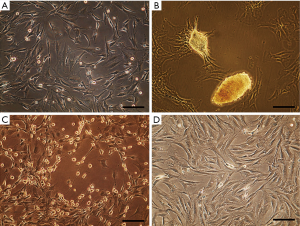
In test group, isolated cells exhibited heterogeneous morphologies after several days as some of them displayed typical fibroblastic morphology (Figure 1A), others grew as spontaneous nodule-like structures (Figure 1B) or showed a spherical shape with extended processes (Figure 1C). In control cultures, cells mostly possessed fibroblastic morphology (Figure 1D).
Evaluation of proteins markers
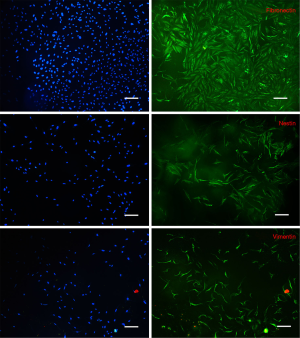
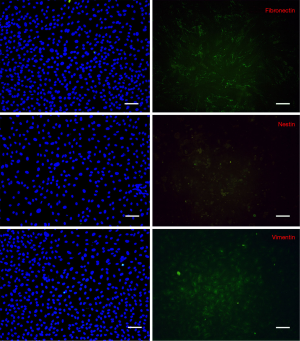
Immunofluorescence examination revealed that the intermediate protein expression patterns of isolated cells were similar to the work of Toma et al. (1), namely positive for vimentin, nestin and fibronectin (Figure 2). In contrast, in control cultures, cells were absolutely positive for vimentin, weakly positive for fibronectin whereas they did not express nestin (Figure 3).
Differentiation potentials assay
Immunofluorescence analysis of neurogenic-induced cells showed positive staining for both neuron-specific β-tubulin III and the astrocyte specific marker, glial fibrillary acidic protein (GFAP) (Figure 4). The proteins were correctly presented and suggest that our induction protocol is successful at producing neural-like cells, both glial and neuronal phenotypes. The control cells, however, demonstrated no visible staining for either of these proteins (images not shown). Additionally, to estimate the percentage of cells that differentiated into neurons and glial cells, random fields of cells from three different experiments were quantitated to determine the total number of cells (as determined by counting DAPI-positive nuclei) versus the total number of βIII-tubulin- and GFAP-positive cells. This analysis revealed that 10.4%±0.2% and 14.3%±0.3% of the cells in these experiments were differentiated into neuronal and glial cells, respectively.
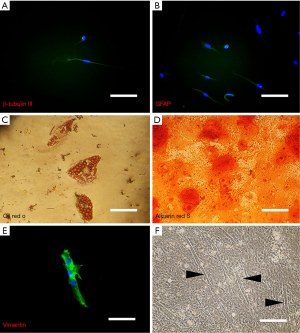
Small oil droplets appeared gradually in the cytoplasm of isolated SKPs about 7–10 days after induction and positively stained with Oil Red O about 14 days after induction. Adipogenic differentiation rate was highly variable, ranging from 1% to greater than 23%.
Under appropriate induction conditions, isolated SKPs were able to differentiate into osteogenic lineage as visualized by alizarin res S about 14 days after induction. The first sign of osteogenic differentiation was appeared on day 7 after culturing in differentiating medium. The increasing accumulation of mineralized matrix was observed with the longer induction period while it reached to its maximal state (between 60−90%) after 15 days (Figure 4).
By culturing SKPs on collagen-coated culture dish and in the presence of proliferation medium, they generated a small subpopulation of muscle cells, as judged by expression of myogenin and typical morphology after 3 weeks under induction (Figure 4).
Discussion
In this research, skin NPCs were isolated and expanded thoroughly by monolayer adherent culture technique. Generally, adult NPCs can be expanded in vitro using two different culture forms: as neurospheres, non-adherent spherical clusters of cells, or as adherent monolayer cultures (8). Neurospheres is suggested to provide a microenvironment that allow the precursor cells to survive in non-physiological conditions in vitro (9). However, it is not suitable for in vitro isolation and expansion of the stem cells and has many problems that have been stated in previous works: (I) cellular heterogeneity within the neurosphere (5); (II) low efficiency of secondary sphere formation from dissociated single cells; (III) a tendency of floating cells to aggregate (6,7,10). Consequently, important modifications to the protocol have been suggested (5,11). Therefore, adherent monolayer cultivation can be used as a suitable alternative to solve the problems associated with neurospheres. This culture form has two main advantages: (I) it provides a more homogeneous undifferentiated population of precursor cells as the cells are uniformly exposed to growth factors in culture medium. This finding was described before for neural stem cells derived from pluripotent mouse embryonic stem (ES) cells and cortical neural stem cell lines from mouse fetuses (E16.5) (12). (II) Direct monitoring of cells is another advantage of monolayer culture which is very important in stem cells biology.
The present study contains three main findings: (I) SKPs as skin-NPCs were enriched thoroughly by changing culture conditions from dermal cell populations; (II) The enriched cells had the expression patterns of intermediate proteins of SKPs, i.e., nestin, vimentin and fibronectin as previously explained; (III) They also could differentiate into ectodermal (neuron, glial cells and keratinocyte (data not shown)) and mesenchymal (adipocyte, osteoblast and myoblasts) lineages. Altogether, the present protocol was designed based on previous works in the field of neural stem/precursor cells isolation and propagation: the initial step of cell expansion by serum which is necessary for low cell yield after dermal digestion and the second expansion step of skin-NPCs by epidermal growth factor (EGF)/basic fibroblast growth factor (bFGF) alone (4,13-15).
Toma and the colleague isolated SKPs by method similar to that used to culture CNS neural stem cells (16). When skin cells of neonatal and adult rodents were dissociated to single cells and grown in suspension culture in the presence of the mitogens FGF2 and EGF, floating spheres of proliferating cells were generated (1). These spheres were positive for nestin, vimentin and fibronectin, markers of both neural and mesenchymal stem cells. Moreover, differentiation of SKPs in vitro resulted in the de novo generation of separate subpopulations of cells expressing neuronal, glial, smooth muscle and adipocyte markers (1,3).
Babu et al. innovated a new protocol with some modification based on their original publication from 2007 for isolation and enriched monolayer cultivation of neural precursor cells from mouse dentate gyrus. They claimed that this method was useful to transfer neurospheres to monolayer culture and for isolating and expanding precursor cells from other brain regions (8). They also mentioned some advantages for isolated NPCs on the basis of their new method as follows: preservation of the criteria of “stemness” in the isolated NPCs, unlimited capacity for self-renewal and differentiation, useful tool to study intrinsic regulatory mechanisms of neural precursor cells, no changes in isolated NPCs with regard to morphology, self-renewal, or molecular profiling (4,8). Being consistent with our data, adherent culture on tissue culture plate or substrate have been shown to be effective on proliferation of neural progenitor cells and drive their selective and rapid expansion (4,8,13,17). In addition, it was indicated that NC-derived stem cells like SKPs could be expanded adherently in the presence or absence of serum after isolation as neurospheres while both manners could significantly increase the expression of the neural progenitor/immature neuron markers nestin and increase their expansion (13,14). However, researches have demonstrated that EGF/bFGF combination directly influence behaviors of neural stem cells like their selective attachment or expansion, gene expression and proliferation. bFGF belongs to the heparin-binding growth factor family. EGF participates in tissue repair and cellular viability in the central nervous system (18-20). Previous evidence has indicated that EGF and bFGF are effective cell mitogens, and have been added to the media of stem cell cultures at different concentrations (21-24). Interestingly, it was also shown that EGF and bFGF treatment enhances neural specification, commitment and differentiation of mesenchymal stem cells and more importantly, decrease their ability to differentiate into mesodermal lineage (25,26). Taken together, these studies are consistent with our finding in regard to the promoting effect of monolayer culture with or without serum in the presence of EGF/bFGF supplementation on selective growth and expansion of isolated skin-NPCs by adherent culture.
Traditionally, SKPs were isolated as neurospheres by culturing digested dermis in proliferation medium supplemented with FGF2, EGF and B27 (1-3). Spheres in suspension were passaged at approximately 6 weeks after isolation. It was shown previously that this culture system resulted in limited expansion (27). Therefore, the researchers investigated an alternative method of cell expansion. They could isolate SKPs in suspension and after a period in order to speed up the expansion rate, propagated them in the presence of serum and EGF/bFGF2. They noticed that the most serum-expanded SKPs were positive for fibronectin (88/3%) and vimentin (84/7%) (27). These findings lend support to dermis-derived cells having some of the differentiation potential as mesenchymal cells. Of note, cells passaged in serum no longer expressed nestin. In contrast, we isolated and expanded SKPs by adherent monolayer culture system thoroughly and finally, we showed that they co-expressed fibronectin, vimentin and nestin.
In another study, the researchers isolated dermal multipotent stem cells (dMSCs) by culturing digested dermis in proliferation medium containing 10% FBS as adherent monolayer culture (28). They found that dMSCs was positive for vimentin and fibronectin, weakly positive for cytokeratin, and negative for nestin. There are some differences between dMSCs and traditional SKPs. Of the intermediate proteins, dMSCs and SKPs have different kinds of intermediate protein expression. As revealed in the literature, dMSCs were positive for vimentin, and negative for nestin, indicating that their mesenchymal origin (28). SKPs, however, were negative for cytokeratin, but positive for nestin and fibronectin, which are typical antigen markers of NC-derived cells in skin (1-3). In contrast, SKPs enriched by our protocol expressed the same intermediate proteins as SKPs isolated by conventional suspension sphere culture.
In conclusion, it is clear that isolating SKPs by such culture conditions may be more beneficial than isolating those as conventional neurospheres from different aspects such as saving times, fast isolation and propagation to obtain large amounts of cells for using in tissue engineering. Of course, in future, we intend to investigate more on selective growth of skin-derived neural precursors and getting rid of fibroblasts by derivational media and other cultural techniques.
Acknowledgements
This work was conducted at Cellular and Molecular Research Center and funded by Ahvaz Jundishapur University of Medical Sciences (AJUMS), Ahvaz, Iran.
Footnote
Conflict of Interests: The authors have no conflicts of interest to declare.
References
- Toma JG, Akhavan M, Fernandes KJ, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 2001;3:778-84. [Crossref] [PubMed]
- Fernandes KJ, McKenzie IA, Mill P, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol 2004;6:1082-93. [Crossref] [PubMed]
- Fernandes KJ, Toma JG, Miller FD. Multipotent skin-derived precursors: adult neural crest-related precursors with therapeutic potential. Philos Trans R Soc Lond B Biol Sci 2008;363:185-98. [Crossref] [PubMed]
- Babu H, Claasen JH, Kannan S, et al. A protocol for isolation and enriched monolayer cultivation of neural precursor cells from mouse dentate gyrus. Front Neurosci 2011;5:89. [Crossref] [PubMed]
- Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods 2005;2:333-6. [Crossref] [PubMed]
- Jessberger S, Clemenson GD Jr, Gage FH. Spontaneous fusion and nonclonal growth of adult neural stem cells. Stem Cells 2007;25:871-4. [Crossref] [PubMed]
- Marshall GP 2nd, Reynolds BA, Laywell ED. Using the neurosphere assay to quantify neural stem cells in vivo. Curr Pharm Biotechnol 2007;8:141-5. [Crossref] [PubMed]
- Babu H, Cheung G, Kettenmann H, et al. Enriched monolayer precursor cell cultures from micro-dissected adult mouse dentate gyrus yield functional granule cell-like neurons. PLoS One 2007;2:e388. [Crossref] [PubMed]
- Bez A, Corsini E, Curti D, et al. Neurosphere and neurosphere-forming cells: morphological and ultrastructural characterization. Brain Res 2003;993:18-29. [Crossref] [PubMed]
- Jensen JB, Parmar M. Strengths and limitations of the neurosphere culture system. Mol Neurobiol 2006;34:153-61. [Crossref] [PubMed]
- Rietze RL, Reynolds BA. Neural stem cell isolation and characterization. Methods Enzymol 2006;419:3-23. [Crossref] [PubMed]
- Conti L, Cattaneo E. Controlling neural stem cell division within the adult subventricular zone: an APPealing job. Trends Neurosci 2005;28:57-9. [Crossref] [PubMed]
- Fortino VR, Chen RS, Pelaez D, et al. Neurogenesis of neural crest-derived periodontal ligament stem cells by EGF and bFGF. J Cell Physiol 2014;229:479-88. [Crossref] [PubMed]
- Bonnamain V, Thinard R, Sergent-Tanguy S, et al. Human dental pulp stem cells cultured in serum-free supplemented medium. Front Physiol 2013;4:357. [Crossref] [PubMed]
- Valenzuela MJ, Dean SK, Sachdev P, et al. Neural precursors from canine skin: a new direction for testing autologous cell replacement in the brain. Stem Cells Dev 2008;17:1087-94. [Crossref] [PubMed]
- Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 1992;255:1707-10. [Crossref] [PubMed]
- Konagaya S, Kato K, Nakaji-Hirabayashi T, et al. Selective and rapid expansion of human neural progenitor cells on substrates with terminally anchored growth factors. Biomaterials 2013;34:6008-14. [Crossref] [PubMed]
- Schmidt A, Ladage D, Schinköthe T, et al. Basic fibroblast growth factor controls migration in human mesenchymal stem cells. Stem Cells 2006;24:1750-8. [Crossref] [PubMed]
- Skaletz-Rorowski A, Eschert H, Leng J, et al. PKC delta-induced activation of MAPK pathway is required for bFGF-stimulated proliferation of coronary smooth muscle cells. Cardiovasc Res 2005;67:142-50. [Crossref] [PubMed]
- Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci 2005;118:4495-509. [Crossref] [PubMed]
- Ito T, Sawada R, Fujiwara Y, et al. FGF-2 suppresses cellular senescence of human mesenchymal stem cells by down-regulation of TGF-beta2. Biochem Biophys Res Commun 2007;359:108-14. [Crossref] [PubMed]
- Kelly CM, Tyers P, Borg MT, et al. EGF and FGF-2 responsiveness of rat and mouse neural precursors derived from the embryonic CNS. Brain Res Bull 2005;68:83-94. [Crossref] [PubMed]
- Quarto N, Longaker MT. FGF-2 inhibits osteogenesis in mouse adipose tissue-derived stromal cells and sustains their proliferative and osteogenic potential state. Tissue Eng 2006;12:1405-18. [Crossref] [PubMed]
- Wang Y, Xu C, Wang H, et al. Efficient derivation of human embryonic stem cell lines from discarded embryos through increases in the concentration of basic fibroblast growth factor. Hum Cell 2012;25:16-23. [Crossref] [PubMed]
- Hu F, Wang X, Liang G, et al. Effects of epidermal growth factor and basic fibroblast growth factor on the proliferation and osteogenic and neural differentiation of adipose-derived stem cells. Cell Reprogram 2013;15:224-32. [PubMed]
- Delcroix GJ, Curtis KM, Schiller PC, et al. EGF and bFGF pre-treatment enhances neural specification and the response to neuronal commitment of MIAMI cells. Differentiation 2010;80:213-27. [Crossref] [PubMed]
- Joannides A, Gaughwin P, Schwiening C, et al. Efficient generation of neural precursors from adult human skin: astrocytes promote neurogenesis from skin-derived stem cells. Lancet 2004;364:172-8. [Crossref] [PubMed]
- Zong Z, Li N, Ran X, et al. Isolation and characterization of two kinds of stem cells from the same human skin back sample with therapeutic potential in spinal cord injury. PLoS One 2012;7:e50222. [Crossref] [PubMed]
Cite this article as: Bayati V, Gazor R, Nejatbakhsh R, Negad Dehbashi F. Enrichment of skin-derived neural precursor cells from dermal cell populations by altering culture conditions. Stem Cell Investig 2016;3:83.


