The Vox mRNA and protein expression in zebrafish Pou5f3 MZspg mutant embryos
Introduction
Pluripotency is an ability of a cell to differentiate into any embryonic tissue. In vertebrata, the property of pluripotency is inherent for every cell of early embryos (at least up to gastrulation). All the pluripotent types of cells, including the embryonic stem cells (ESC), express the transcription factors: Pou5f3 (outdated Pou5f1/Oct4), Sox2 and Nanog, which play a principal role in maintaining the pluripotency. They are called “transcription factors of pluripotency”. These key factors control the expression of thousands of genes in ESC (1-4). In zebrafish embryos, 12–15% of genes become active in quick and coordinated manner (5) at the time of zygotic genome activation (ZGA) beginning after 10 cell divisions. The role of genome global activators in zebrafish is performed by several transcription factors—homologues of the transcription factors of pluripotency—Pou5f3, SoxB1 and Nanog (6,7). Under their influence there are activated tissue-specific genes in different parts of embryo (4,8,9). In particular, it was shown that in the MZspg (spiel ohne grenzen) mutants devoid of both maternal and embryonic Pou5f3 functions, the expression of 595 genes was almost halved (8). Pou5f3 in the embryo acts as an activator of gene expression determining ventral fates by direct activation of the Vox promoter, and this is a phylogenetically conserved mechanism. In wild type embryos, the Vox expression was enhanced by the Pou5f3, but it was partially suppressed in the mutants (10). However, the gene expression was estimated only on the level of the mRNA synthesis. Here we investigated the expression of Vox and Vent mRNA and encoded proteins in the WT and mutant embryos.
Methods
Embryos, stages
Zebrafish (Danio reria) developmental stages were identified according to tables (11). We used the embryos during the Cleavage (8-64 cells), Blastula (sphere) and Gastrula (shield) periods.
Production of antibodies
Rabbit polyclonal antibodies against zebrafish Vox and Vent were produced by Almabion Company (Russia) using peptides pvldvqepekktrphvpc and skfsvewlsqsfhdqekc, respectively. Both antibodies were purified by affinity chromatography and conjugated with horseradish peroxidase. The same conjugate with rabbit polyclonal antibodies against human immunoglobulin (Imtek, Russia) was used in control experiments.
Whole-mount immunostaining of embryos and Western blotting
Embryos were fixed according to Klymkowsky lab manual (12) in MEMFA solution followed by 20% DMSO—80% methanol (Dent’s fixative) and then whole-mount immunostaining was carried out according to standard procedure with slight modifications (13). Western-blot analyses were performed with single appropriate HRP-conjugated antibodies using SuperSignal Western Blot Enhancer Kit (Thermo Scientific, USA) according the manufacturer recommendations.
Whole-mount in situ hybridization
Whole-mount in situ hybridization was performed by a standard procedure (14). Plasmids pBS-SK carrying Vox cDNA and pCS2+ carrying Vent cDNA were used for synthesizing of digoxigenin-RNAs (Roche) by T3 RNA polymerase (Fermentas).
Results and discussion
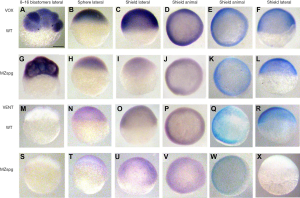
Here we present our results on spatiotemporal distribution of the Vox and Vent mRNAs and proteins in zebrafish WT and MZspg793 mutant embryos at early developmental stages (Figure 1). We suppose that the Vox mRNA revealed at the stage of 8-16 blastomeres in both WT and mutants had been maternally prestored, then after the ZGA the new-synthesized mRNAs appeared only in the WT. The MZspg mutants failed to synthesize it de novo and the prestored molecules gradually degraded. Whole-mount immunostaining with the anti-Vox antibodies could not reveal any difference in staining of the WT and mutant embryos at the gastrula (shield stage) (Figure 1E,F,K,L).
These results were confirmed with the Western-blot analysis of protein extracts of the WT and mutant embryos (Figure 2).
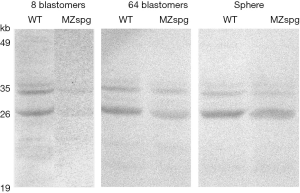
At the stage of 8–16 blastomeres, there are seen several protein bands (theoretically calculated the Vox protein molecular mass is 28 kDa) recognized by the anti-Vox antibodies in the case of the WT, and only slight shadows on the track with the extract of the mutants. At the later stages (64 cells and sphere) the Western blotting revealed the increasing amount of the Vox protein in mutants. It became almost comparable with that in the WT. Hence, at these stages the Vox protein is actively synthesized on the disappearing mRNAs. Interestingly, the Vox protein migrated as a doublet. This situation had been already described (15). The 35S-labeled proteins were translated in vitro from capped mRNA. The authors noted that Vox and Pou5f1 artifactually migrated in electrophoresis as a doublet. However, Lippok and coauthors (16) investigated zebrafish Pou5f1 posttranslational modifications, and found dynamic patterns of phosphorylation during development. Pou5f1 in Western blots showed several discrete bands. The higher molecular mass appeared to be predominantly caused by phosphorylation. During gastrulation stages, the higher molecular mass forms of Pou5f1 prevailed. The authors proposed the possibility that Pou5f1 function may get modulated posttranslationally by phosphorylation, and that embryonic signaling pathways may contribute globally or in a region-specific manner to control of Pou5f1 activity. We suppose the doublet Vox band not to be an artifact but, like that of Pou5f1, it shows differently modified forms.
The Vent mRNA whole-mount in situ hybridization analysis of the WT and mutant zebrafish embryos revealed no sufficient differences. At the stage of 8–16 blastomeres there was no Vent mRNA staining in either WT or MZ embryos (Figure 1M,S). Then it appeared as a result of the ZGA at the sphere and shield stages. Perhaps, it seems to be slightly weaker in MZ embryos (Figure 1N,O,T,U). The whole-mount immunostaining by the anti-Vent antibodies also could not reveal any difference in staining of the WT and mutant embryos (Figure 1Q-W). The Western blot with anti-Vent antibodies revealed faint Vent double bands in the WT and MZ (data not shown).
Summarizing the results obtained we can see that Vox mRNA is maternally stored and can be revealed at the 8-16 blastomeres stage in both WT and MZ embryos. After ZGA in the WT embryos the new Vox mRNA was synthesized. The MZspg mutants lacking Pou5f3 failed to synthesize the new Vox mRNA while the maternally prestored mRNA was degrading. Hence, the maternal synthesis of Vox mRNA did not depend on the Pou5f3 but the zygotic synthesis did. The Vox protein in WT seemed to be prestored or/and synthesized on the maternal and zygotic Vox mRNA. In MZspg mutant, Vox protein had not been prestored maternally but was synthesized on the stored Vox mRNA. Actually, the amount of the protein does not depend directly on the amount of its mRNA. There are many factors affecting the rate of protein synthesis. Concerning the Vent mRNA, we can say it was not maternally prestored and then its synthesis slightly depended on the Pou5f3. This suggestion was confirmed by almost similar data of the whole-mount immunostaining of the WT and MZspg embryos. Our results do not contradict with the idea of Belting and coauthors (10), who concluded that the zygotic Vox was a direct transcriptional target of Pou5f3, while Vent was upregulated to a lesser extent. Pou5f3 is not a single regulator of Vent family gens. It was also shown (17) that the zygotic synthesis of Vox and Vent mRNA were regulated by maternal Runx2, a transcription factor essential for bone formation. The data obtained suggest the existence of mechanism sustaining a required Vox and Vent proteins level, but this mechanism was not directly dependent on the Pou5f3. The regulation of translation, posttranslational modifications (16) and direct protein-protein interactions (15) may play a role in this process.
Acknowledgements
We would like to thank Dr. D. Onichtchouk for zebrafish embryos (MZspg793 and WT) and plasmids kindly donated.
Funding: This study was supported by grant Russian Foundation for Basic Research (14-54 12008).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kim J, Chu J, Shen X, et al. An extended transcriptional network for pluripotency of embryonic stem cells. Cell 2008;132:1049-61. [Crossref] [PubMed]
- Boyer LA, Lee TI, Cole MF, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005;122:947-56. [Crossref] [PubMed]
- Chen X, Xu H, Yuan P, et al. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell 2008;133:1106-17. [Crossref] [PubMed]
- Onichtchouk DV, Voronina AS. Regulation of Zygotic Genome and Cellular Pluripotency. Biochemistry (Mosc) 2015;80:1723-33. [Crossref] [PubMed]
- Tadros W, Lipshitz HD. The maternal-to-zygotic transition: a play in two acts. Development 2009;136:3033-42. [Crossref] [PubMed]
- Lee MT, Bonneau AR, Takacs CM, et al. Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition. Nature 2013;503:360-4. [Crossref] [PubMed]
- Leichsenring M, Maes J, Mössner R, et al. Pou5f1 transcription factor controls zygotic gene activation in vertebrates. Science 2013;341:1005-9. [Crossref] [PubMed]
- Onichtchouk D, Geier F, Polok B, et al. Zebrafish Pou5f1-dependent transcriptional networks in temporal control of early development. Mol Syst Biol 2010;6:354. [Crossref] [PubMed]
- Onichtchouk D. Pou5f1/oct4 in pluripotency control: insights from zebrafish. Genesis 2012;50:75-85. [Crossref] [PubMed]
- Belting HG, Wendik B, Lunde K, et al. Pou5f1 contributes to dorsoventral patterning by positive regulation of vox and modulation of fgf8a expression. Dev Biol 2011;356:323-36. [Crossref] [PubMed]
- Kimmel CB, Ballard WW, Kimmel SR, et al. Stages of embryonic development of the zebrafish. Dev Dyn 1995;203:253-310. [Crossref] [PubMed]
- Dent JA, Polson AG, Klymkowsky MW. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development 1989;105:61-74. [PubMed]
- Pshennikova E, Goncharenko A, Voronina A. The XVent-2 protein expression in multipotent cells of the neural crest in tails of Xenopus laevis and Rana temporaria tadpoles. Indian Journal of Fundamental and Applied Life Sciences 2014;4:180-7.
- Lehmann R, Tautz D. In situ hybridization to RNA. Methods Cell Biol 1994;44:575-98. [Crossref] [PubMed]
- Zhao J, Lambert G, Meijer AH, et al. The transcription factor Vox represses endoderm development by interacting with Casanova and Pou2. Development 2013;140:1090-9. [Crossref] [PubMed]
- Lippok B, Song S, Driever W. Pou5f1 protein expression and posttranslational modification during early zebrafish development. Dev Dyn 2014;243:468-77. [Crossref] [PubMed]
- Flores MV, Lam EY, Crosier KE, et al. Osteogenic transcription factor Runx2 is a maternal determinant of dorsoventral patterning in zebrafish. Nat Cell Biol 2008;10:346-52. [Crossref] [PubMed]
Cite this article as: Voronina A, Pshennikova E. The Vox mRNA and protein expression in zebrafish Pou5f3 MZspg mutant embryos. Stem Cell Investig 2016;3:79.


