
Current status and prospects of regenerative medicine for spinal cord injury using human induced pluripotent stem cells: a review
Introduction
Spinal cord injury (SCI) is damage to the spinal cord resulting from trauma or other conditions such as infections and vascular dysregulation (1). In SCI patients, damage in motor, sensory, and autonomic neurons lead to paralysis and numbness (2). Globally, the number of incident cases of total SCI was estimated to be 0.9 million in 2019 for both sexes (3). The number is expected to increase with the aging population in developed countries, as elderly people are less resistant to damage, even by minor traumatic injuries, putting them at risk of developing SCI, alongside with rising number of cases of incomplete paralysis in the elderly, caused by falls in daily lives (2).
Currently, there is no radical treatment for SCI patients (4), leaving the only valid treatment option, the surgical intervention, it enables relieving pressure on the spinal cord caused by trauma, repositioning or stabilizing a dislocated loose spine, and subsequent rehabilitation. This treatment is intended to preserve residual neurological function, but not to provide recovery of the lost neuronal dysfunction, especially in patients with severe paralysis (4). Therefore, the development of new treatments has been highly anticipated with huge demand. In effort to resolve the situation, numbers of ongoing clinical trials that were based on various strategies have been established, including neuroprotective/neurotrophic factors, repulsive guidance molecules, and cell replenishment.
In this review, we will initially outline the novel therapies currently under development for SCI, followed by focusing on cell-based regenerative medicine, which will have the potential to attract much attention in the future. Finally, we will also introduce ongoing first-in-human clinical research in subacute SCI in Japan.
Ongoing approaches for treating SCI
To achieve effective therapies for SCI patients, we should pay attention to the pathophysiology of SCI, which varies greatly depending on the phase after injury (5). During the acute stage, it can last several days after injury, strong hemorrhagic and inflammatory responses occur at the site of injury, hence anti-inflammatory and neuroprotective approaches are selected when treating (4). In addition, factors expected to prevent inflammation and neuronal damage, including hepatocyte growth factor (HGF) and granulocyte colony stimulating factor (G-CSF), are being tested in clinical trials (6,7) (Table 1). However, because these treatments are intended to slow the progression of the injury rather than replace lost neuronal function, the development of radical treatments is desired.
Table 1
| Phase | Name | Stage | Company | Country/region | Drug type | Mechanism of action | Registry number |
|---|---|---|---|---|---|---|---|
| Phase 3 | Neuro-Spinal Scaffold | All | InVivo Therapeutics | UK/US | Bioresorbable polymer | Neuroprotectant | NCT03762655 |
| Phase 3 | KP-100IT | Acute | Kringle Pharma | Japan | Human HGF | Nutrition effect | NCT04475224 |
| Phase 3 | G-CSF | Acute | Chiba University | Japan | Human G-CSF | Neuroprotectant | UMIN000018752 |
| Phase 2 | AXER-204 | Chronic | ReNetX Bio | US | Nogo-R decoy | Neuroprotectant | NCT03989440 |
| Phase 2 | Elezanumab | Acute | AbbVie | US/Canada/Japan/Australia/Spain | Human anti-RGMa mAbs | Neuroprotectant and neurorestoration | NCT04295538 |
| Phase 2 | MT-3921 | Acute | Mitsubishi Tanabe Pharma | US/Canada/Japan | Human anti-RGMa mAbs | Neuroprotectant | NCT04683848 |
| Phase 2 | PMZ-1620 | Acute | Pharmazz | India | Endothelin B agonists | Neuroprotectant | NCT04054414 |
| Phase 2 | NG-101 | Acute | Novartis | Europe | anti-Nogo-A mAbs | Neuroprotectant | NCT03935321 |
| Phase 2 | BA-210 | Acute | BioAxone Biosciences | US | Rho GTPase inhibitor | Neuroprotectant | NCT02053883 |
| Phase 1 | NVG-291 | All | NervGen Pharma | Australia | Protein tyrosine phosphatase sigma inhibitor | Neuroprotectant | NCT05308953 |
| Phase 1 | ALMB-0166 | Acute | AlaMab Therapeutics | Australia | Gap junction alpha-1 protein inhibitor | Neuroprotectant | NCT05524103 |
HGF, hepatocyte growth factor; G-CSF, granulocyte colony stimulating factor; RGM, repulsive guidance molecule; mAb, monoclonal antibody.
The inflammatory response subsides over the subacute period, which is considered the optimal time frame for cell therapies (8), a recently introduced treatment option expected to be a breakthrough in treating SCI (9). There are a series of ongoing clinical studies for establishing cell therapy for SCI (Table 2). One class of SCI cell therapy employs a type of somatic stem cell called mesenchymal stem cells (MSCs) (10) that provide nutrition effects leading to neuroprotection and release various growth factors to improve the local environment of the injury site (10). Since the MSCs eventually disappear from the patient after the administration, the treatments by MSC products aim to ameliorate damaged spinal cord function through trophic actions rather than recovery by engraftment of administrated cells (10). MSCs possess different characteristics depending on their origin, derivation can originate from bone marrow, adipose tissue, and umbilical cord, which are also developed as sources of cell-based therapy (11). Although there is limited information on outcomes after transplantation into the patients, the potential mechanistic insight into how the MSCs include beneficial effects includes trophic, neuroprotective, and anti-inflammatory effects (Table 2). Since MSCs have the property of migrating to injured sites, they can be administered by intravenous injection, which may have the advantage of being less invasive to patients (12). However, since MSCs eventually disappear from the patient after the administration, further long-term follow-up is required to determine whether the functional recovery is permanent rather than transient. One of the MSC products, called Stemirac©, has already been conditionally approved by the early authorization system in Japan (Table 2). In the Stemirac© Phase 2 study, 13 SCI patients received a single infusion of autologous MSCs and were evaluated for their safety and neurological function (12). While no serious adverse events were reported after the injection of MSCs, neurological improvement was observed in 12 of the 13 patients (12). Further evaluations are awaited for the realization of the cell-based therapy for SCI patients using Stemirac©. SCI treatment with neural stem/progenitor cells (NS/PCs) has been actively pursued as another type of cell therapy (13). In these therapies, the NS/PCs are injected into the injured spinal cord to replenish the cells, restore and maintain the spinal cord function for extended periods (13). Therapeutic intervention using several types of NS/PCs are in clinical trial stages, such as AST-OPC1 [embryonic stem cells-origin (14,15)] and NSI-566 [fetal spinal cord-origin (16)] (Table 2). However, using NS/PCs derived from human embryonic stem cells or isolated from fetuses may pose some ethical concerns (13).
Table 2
| Phase | Name | Stage | Company | Country/region | Cell origin | Drug type | Mechanism of action | Registry number |
|---|---|---|---|---|---|---|---|---|
| Launched | Stemirac© | Subacute | Nipro | Japan | Autologous | BM-MSCs | Nutrition effect | JMA-IIA00154 |
| Phase 2 | MC-001 | Chronic | StemCyte | US/Taiwan/China | Allogeneic | UCBMNCs | CNS modulator | NCT03979742 |
| Phase 2 | MSCs | Subacute/Acute | Mayo Clinic | US | Autologous | AD-MSCs | Nutrition effect | NCT04520373 |
| Phase 2 | CL2020 | Subacute/Acute | Life Science Institute | Japan | Allogeneic | Muse cells | Tissue repair | jRCT1080224764 |
| Phase 2 | FAB-117-HC | Acute | Ferrer Internacional | Spain | Allogeneic | AD-MSCs | Neuroprotectant | NCT02917291 |
| Phase 1/2 | AST-OPC1 | Subacute | Lineage Cell Therapeutics | US | Allogeneic | OPC derived from ESC | OPC replacement | NCT02302157 |
| Phase 1 | AlloRx | All | Vitro Diagnostics | Antigua and Barbuda | Allogeneic | UC-MSCs | Anti inflammatory | NCT05152290 |
| Phase 1 | MSCs | Chronic | University of Jordan | Jordan | Autologous | BM-MSCs | Neuroprotectant | NCT04288934 |
| Phase 1 | NSI-566 | Chronic | Seneca Biopharma | US | Allogeneic | NPCs derived from fetal SC | Neuroprotectant | NCT01772810 |
| Phase 1 | NPCs | Subacute | Keio University | Japan | Allogeneic | NPCs derived from iPSC | Neuroprotectant | UMIN000035074, jRCTa031190228 |
BM-MSC, bone marrow mesenchymal stem cell; UCBMNCs, umbilical cord blood mononuclear stem cells; MSC, mesenchymal stem cell; AD-MSC, adipose mesenchymal stem cell; OPC, oligodendrocyte precursor cell; ESC, embryonic stem cell; UC-MSC, umbilical cord-derived mesenchymal stem cell; NPC, neuronal progenitor cell; SC, spinal cord; iPSC, induced pluripotent stem cell.
Induced pluripotent stem cells (iPSCs) as a realistic source for regenerative medicine
In 2007, Takahashi and colleagues established human iPSCs (17), providing a way to circumvent the above-mentioned ethical issues. iPSCs are generated by introducing a cocktail of reprogramming factors into somatic cells such as skin fibroblasts or lymphoid cells (17,18). The iPSCs were considered as a superior choice over other cell sources due to several advantages, including the fact that autologous transplantation therapies may become a viable option for regenerative medicine (9). Since iPSCs will be originated from patients, the immune rejection after the transplantation is theoretically negligible (9).
To apply the same concept, immune rejection can be minimized by taking advantage of human leukocyte antigen (HLA)-matched donor iPSC-derived cells (19). In Japan, an iPSC stock project led by Kyoto University Center for iPS Cell Research and Application (CiRA) is underway for applying HLA-matched allogeneic transplantation (19), and HLA homozygous iPSCs that match a large population have been established (19,20). The development of universal cells in which HLA is partially deleted by genome editing is underway to cover a more significant number of patients (21). Using these cells as sources for treatment not only limits the risk of immune rejection but is also able to reach across a wide range of populations for clinical applications, with a reasonable price.
Cell transplantation therapy using iPSC-derived NS/PCs
Since NS/PCs can then be prepared in vitro from iPSCs, researchers have great interest in iPSC-derived NS/PCs (iPSC-NS/PCs) to administer them to the injured spinal cord of SCI patients (8). Several pre-clinical evaluations have been initiated, and there are a series of reports demonstrating the transplantation of human iPSC-NS/PCs into the injured spinal cord of subacute SCI in rodent and non-human primate models (22,23). In these reports, the transplanted iPSC-NS/PCs are shown to display an ability to be engrafted into the host spinal cord and differentiated into three neuronal lineages, i.e., neurons, astrocytes, and oligodendrocytes (22,23). Notably, the transplanted iPSC-NS/PCs successfully trigger functional recovery by neurological and electrophysiological tests, implying the reconstruction of neuronal circuits by the grafts (22). In addition, using a synaptic tracing technique, Fujimoto and colleagues demonstrated functional recovery of hind limb motor function by reconstructing the corticospinal tract in a relay fashion by iPSC-NS/PC-derived neurons using synaptic tracing (24). Thus, it is conceivable that motor recovery in SCI animals is achieved by replacing lost neurons with the neurons derived from the iPSC-NS/PCs after transplantation.
Mechanistic insight into how iPSC-NS/PCs trigger functional recovery
It was unclear how the neuronal activity of transplanted cells contributes to the locomotor functional recovery after engraftment. However, recent reports shed light on this issue using a technology called a designer receptor, which is exclusively activated by designer drugs (DREADD) systems (25,26). In this chemogenetic system, the neurons which express a designer receptor can be either selectively activated or inhibited when treated by a designer drug (27).
We prepared the iPSC-NS/PCs devised to express inhibitory DREADD M4 to examine whether inhibition of neuronal activity in the grafted neurons impacts the functional motor recovery in the SCI animal model (25). We transplanted engineered iPSC-NS/PCs into the spinal cord of subacute SCI mice (25). In this study, we initially performed trans-synaptic tracing and revealed the structural integration of graft neurons into the host motor circuitry, once recovery of locomotor function was confirmed after the transplantation of DREAD M4-expressing iPSC-NS/PCs, the prototypical DREADD activator clozapine N-oxide (CNO) was administered to suppress the functional neuronal activity in the graft. Significantly, CNO administration temporarily reduced the locomotor function, indicating that the neurons differentiated from the iPSC-NS/PCs are functionally matured and integrated into the neural circuit of the host spinal cord. Thus, the neuronal activity of iPSC-NS/PC-derived neurons is essential for improving locomotor function.
Another report utilized the DREADD M3 system that selectively enhances the synaptic transmission from the grafted neurons to host neurons (26). In this system, CNO treatment activates neuronal firing in the neurons derived from iPSC-NS/PCs carrying the DREADD M3. The engineered iPSC-NS/PCs expressing this system were transplanted into the spinal cord of the subacute SCI model. The results showed that consecutive and selective stimulation of grafts by the DREADD M3 resulted in enhanced expression of synapse-related genes. Interestingly, in this system, continuous stimulation of the grafts affects the host tissue. They observed enhanced expression of synaptic proteins in surrounding host tissues and prevention of atrophy of the injured spinal cord. These results indicate synapse formation between graft-derived and host spinal cord neurons, which is upregulated by the neuronal activity of graft neurons, and its contribution to improvement in the locomotor function of subacute SCI. These reports elucidated that transplanted cells do engraft in host tissues, differentiate into the neuronal lineage, and connect to the host neurons in an activity-regulated manner to contribute to functional improvement.
Safety management for iPSC-NS/PCs therapy
For regenerative medicine using iPSC-NS/PCs in actual clinical settings, it is necessary to eliminate the risk of tumorigenesis of the transplanted iPSC-NS/PCs by remnant undifferentiated iPSCs or the existence of transformed cells (28).
For the transformation of the iPSC derivatives, we should pay attention to the remaining reprogramming factors activated in the iPSCs, or genetic mutation of iPSCs occurred during the culture of iPSCs (29). Therefore, selecting safe iPSC lines as a source cell line, rigorous process control during differentiation and quality check of intermediate and final products are essential to avoid the risk of tumorigenesis as much as possible (30).
Nevertheless, based on the result of karyotype analysis, the iPSC-NS/PCs with abnormal karyotypes formed tumor-like tissues after transplantation into immunodeficient mice (30). Furthermore, a large number of de novo copy number variations (CNVs) and the increased CNV frequency during the preparation of NS/PCs from iPSCs were suggested to increase the risk of tumorigenesis in vivo (30). Needless to say, the genomic stability of the parental iPSCs is related to the safety of the iPSC-NS/PCs.
In addition, the DNA methylation status in the iPSC-NS/PCs should be considered for safety assurance as suggested by Iida et al., who examined the genome-wide DNA methylation profiles in non-tumorigenic and tumorigenic iPSC-NS/PCs (31). The tumorigenic iPSC-NS/PCs exhibited different DNA methylation profiles from the non-tumorigenic iPSC-NS/PCs, in particular, tumorigenic iPSC-NS/PCs display a more aberrant DNA methylation profile during passages in a particular type of tumor suppressors. Thus, DNA methylation profiles might be a helpful quality control parameter to evaluate the tumorigenicity of iPSC-NS/PCs for clinical use.
The pre-evaluation of iPSC-NS/PC using in vivo system is also essential to estimate the safety of the iPSC-NS/PCs. For example, tumorigenic risk of the iPSC-NS/PCs was assessed by transplanting cells into the spinal cord and/or central nervous system tissues of immunodeficient mice to observe the tumor formation (30), intensive histological evaluation of the graft for an extended period helped estimate the safety of the iPSC-NS/PCs.
Safeguard for iPSC-NS/PCs against tumorigenic overgrowth
Since the NS/PCs hold innate proliferative ability, controlling over-proliferation is crucial after the transplantation (30). Notch signaling plays an essential role in the proliferation of NS/PCs and controls the balance between neuronal and glial differentiation (32,33). γ-secretase inhibitors (GSIs) inhibit Notch signaling, suppress cell proliferation of NS/PCs, and enhance their differentiation into neurons (32,33). Thus, pre-treatment of the GSI on the iPSC-NS/PCs before the transplantation seems desirable to preserve the safety of therapeutic iPSC-NS/PCs. Indeed, when treated by the GSI, iPSC-NS/PCs efficiently differentiated into neurons with limited cell proliferation after the transplantation (34). The expression of mature neuronal marker-related genes increased significantly, whereas that of early neural marker genes and pluripotency/self-renewal marker genes decreased by GSI treatment in vitro. Functional recovery is observed when transplanted into the spinal cord of subacute SCI mice. It is confident to say that the GSI is capable to lower the risk of tumorigenesis, enforcing the safety-lock system. Besides these approaches to actively eliminate the tumorigenic cells from the final product, a system to ablate tumorigenic cells after transplantation is also being studied. The idea of this method is to load transplanted cells with a suicide gene, activation of the system can ablate the graft cells when the cells become tumorigenic (35,36). One of the examples is an inducible caspase-9, which can be transduced into the iPSC-NS/PCs (35). This system is based on human caspase 9 fused with a modified human FK506-binding protein being conditionally dimerized by a small-molecule chemical inducer of dimerization (CID) (37). When exposed to a CID, the inducible caspase 9 becomes activated, leading to the rapid apoptosis of cells expressing this construct (37). In this study, the authors used iPSC-NS/PCs with high-tumor formation potential to test the efficacy of this system (35). As expected, the transplanted iPSC-NS/PCs formed tumors several weeks after transplantation into the injured spinal cord of mice. In due course, a CID activated caspase 9 dimer is injected to the site, leading ablation of the transplanted cells with only a slight loss of motor function. This result suggested the positive effect of this approach (35). However, this method ablated not only graft-derived tumorigenic cells (e.g., undifferentiated or transformed cells) but also adequately differentiated neurons. To solve this problem, another method, the herpes simplex virus type I thymidine kinase (HSV-tk) transgene system, was applied (36). HSV-tk phosphorylates ganciclovir (GCV), an antiviral medicine, and the phosphorylated GCV is toxic to immature/proliferating cells such as tumor cells (38). In this system, the iPSC-NS/PCs were transduced to express the HSV-tk gene and further transplanted into the spinal cord of the SCI animal model. Transplantation of the iPSC-NS/PCs temporarily restored locomotor function after several weeks of differentiation and maturation, which was lost when tumor formation prevails (36). The advantage of this system is the selective ablation of immature/proliferative cells by GCV. Thus, this approach enables the recovery of locomotor function by eliminating the undesired immature/proliferative cells but not post-mitotic mature neurons, which are essential for functional recovery. Since this system can theoretically be used as a curative measure every time a tumorigenic episode occurs, it has the potential as both a preventative and curative measure against tumorigenesis following cell transplantation.
A clinical trial using human iPSC-derived NS/PCs in the subacute stage
The subacute stage of SCI is thought to be the most preferred condition for transplanted cells because inflammatory responses after the injury have mostly subsided and, particularly for cell replenishment therapies, glial scars and cavities that are barriers to cell engraftment and neurite outgrowth have not been developed yet (8). Therefore, most of the cellular medicines currently undergoing clinical trials for SCI are intended for the subacute stage (Table 2), a 2 to 4 weeks period after injury.
In 2020, the first clinical research for subacute SCI using human iPSC-NS/PCs was started in Japan (4,5,9).
In this clinical research, the iPSC strain obtained from the CiRA’s iPSC Stock Project was used as a source cell line (5), and various quality assessments in vitro and long-term safety evaluations in vivo were performed (4). During this open-label, single-arm clinical research, 2 million cells will be transplanted into the spinal cord parenchyma in 4 patients with American Spinal Cord Injury Association Impairment Scale (AIS)-A subacute SCI at 14–28 days post-injury followed by a 1-year observation period. Before transplantation, thawed iPSC-NS/PCs from cryopreserved tubes will be treated with GSI to promote cell differentiation. The focus of the study is safety, and once safety is confirmed, dose escalation from 2 million cells might be considered for better quality-of-life outcomes and functional recovery.
Although the clinical research started in 2020, enrollment was suspended due to the COVID-19 pandemic. Therefore, the first surgery was performed in December 2021. So far, the safety of the iPSC-NS/PCs subjected to the first SCI patient has been monitored by the independent Data Monitoring Committee, which concluded that there would be no problem in continuing the research. The detailed outcome after the iPSC-NS/PCs-based cell therapy for subacute SCI patients will be reported in the future.
Pre-clinical studies using iPSC-NS/PCs in the chronic stage
Most of the cell therapies currently under clinical development target the subacute stage of SCI. However, the majority of SCI patients are in the chronic stage, i.e., one year or more after the injury (6). Therefore, effective treatment of chronic stage patients is highly coveted. The spinal cord of chronic stage patients offers a more unfavorable environment for neuronal regeneration due to glial scar formation and cavitation associated with neuronal cell death and reactive astrocytes (39). For example, the glial scar forms a barrier to axonal extension. A barrier can be formed with glial scars, the surgical removal of the glial scar has been performed in some patients with complete SCI but not without the risk of worsening symptoms, especially in patients with incomplete SCI (40). The efficacy of chondroitinase-ABC (Ch-ABC), which enzymatically degrades chondroitin sulfate proteoglycans, a component of glial scars, has been investigated (41). Additionally, biocompatible scaffold materials, such as collagen, are being developed for filling in a cavity, and some, such as the Neuro-Spinal Scaffold™, are in clinical trials (42). Combining these treatment modalities with cell transplantation might be a promising therapeutic strategy.
It was recently reported that GSI-treated iPSC-NS/PCs showed some efficacy in a rodent chronic model as well as a subacute model (43). Furthermore, axonal regrowth, remyelination, inhibitory synapse formation with the host neural circuitry, and reticulospinal tract fiber formation were observed, indicating the usefulness of the iPSC-NS/PCs even in chronic SCI patients.
During the progression of SCI, the number of demyelinated axons progressively increased up to 450 days after injury (44), thereby leading to loss of neuronal function. In other words, remyelination of residual axons is essential to treat chronic stage SCI. This is in line with the idea that oligodendrocyte replenishment might offer a promising treatment option for chronic incomplete SCI (39). A method to differentiate human iPSCs into oligodendrocyte progenitor-rich NS/PCs has been developed (45,46). Approximately 50% of the oligodendrocyte progenitor-rich NS/PCs were reported to differentiate into oligodendrocytes after transplantation into the spinal cord parenchyma of the subacute SCI model (46).
Furthermore, remyelination of host rodent neuronal axons by the grafts was observed by electron microscopy, indicating such NS/PCs would be helpful in chronic SCI treatment. Notably, the combinatorial therapy of oligodendrocyte progenitor-rich NS/PCs and Ch-ABC also triggered motor function recovery in the rodent chronic SCI model (39). These data indicated that oligodendrocytes differentiated from NS/PCs are essential for achieving effective functional recovery in the chronic stage of SCI.
The road to clinical trials for chronic stage
Clinical studies on cell replacement therapy have been conducted for chronic SCI (Table 2). Considering quality control and cost, clinical trials with allogeneic transplantation will be advanced first, even in the chronic stage. If allotransplantation is confirmed to be safe and effective, autologous transplantation might follow in the future. Regarding target conditions or severity of patients, cell therapy would be advanced first for patients with incomplete SCI. As it may be challenging to achieve desired efficacy by cell transplantation alone for severe conditions such as complete transection, a combination of cell transplantation and scaffold materials that fill the gaps in the transected spinal cord or drugs that dissolve glial scars may be useful.
Conclusions
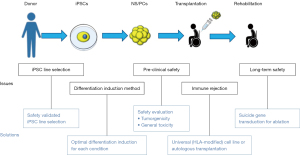
Based on the accumulated non-clinical study data, clinical research for regenerative cell therapy against subacute stage SCI using iPSC-derived NS/PCs has been started. In addition, by elaborating the iPSC differentiation method to produce NS/PCs with optimal differentiation properties, clinical studies against chronic SCI are steadily approaching. As a result, it is hoped that more effective treatments will be developed to cover widely different pathology among SCI patients. Continuous effort and active collaboration between basic research and clinical science are essential to achieve this goal (Figure 1).
Acknowledgments
We sincerely thank the Okano laboratory members in the Department of Physiology of Keio University School of Medicine. We are also grateful to Dr. Narihito Nagoshi, Masaya Nakamura, and all members of the Spinal Cord Research Team of the Department of Orthopedic Surgery, Keio University School of Medicine, for their contribution to the original works described in this review. We apologize for not being able to cite many relevant and excellent papers due to length limitations.
Funding: This work was supported by the Research Center Network for Realization of Regenerative Medicine of the Japan Science and Technology Agency (JST), the Japan Agency for Medical Research and Development (AMED) (Grant No. JP22bm0204001 to HO and Grant No. JP18bm0404022 to JK), and by a medical research grant related to traffic accidents from the General Insurance Association of Japan to JK.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://sci.amegroups.com/article/view/10.21037/sci-2022-037/coif). MI, RY and AI are employees of Sumitomo Pharma Co., Ltd., which conducts research to develop a regenerative medicine for spinal cord injury using human iPSC-derived neural stem/progenitor cells. HO receives grants from Research Center Network for Realization of Regenerative Medicine from Japan Agency for Medical Research and Development (AMED) (Grant No. JP22bm0204001), and he reports stocks and consulting fees from K Pharma, Inc. and SanBio Company Limited. JK serves as an unpaid editorial board member of Stem Cell Investigation from July 2022 to June 2024. Besides, he receives grants from Research Center Network for Realization of Regenerative Medicine from Japan Agency for Medical Research and Development (AMED) (Grant No. JP18bm0404022) and a medical research grant related to traffic accidents from the General Insurance Association of Japan. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDeri
References
- McEntire CR, Dowd RS, Orru' E, et al. Acute Myelopathy: Vascular and Infectious Diseases. Neurol Clin 2021;39:489-512. [Crossref] [PubMed]
- Nagoshi N, Tsuji O, Nakamura M, et al. Cell therapy for spinal cord injury using induced pluripotent stem cells. Regen Ther 2019;11:75-80. [Crossref] [PubMed]
- Ding W, Hu S, Wang P, et al. Spinal Cord Injury: The Global Incidence, Prevalence, and Disability From the Global Burden of Disease Study 2019. Spine (Phila Pa 1976) 2022;47:1532-40. [Crossref] [PubMed]
- Sugai K, Sumida M, Shofuda T, et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: Study protocol. Regen Ther 2021;18:321-33. [Crossref] [PubMed]
- Tsuji O, Sugai K, Yamaguchi R, et al. Concise Review: Laying the Groundwork for a First-In-Human Study of an Induced Pluripotent Stem Cell-Based Intervention for Spinal Cord Injury. Stem Cells 2019;37:6-13. [Crossref] [PubMed]
- Nagoshi N, Tsuji O, Kitamura K, et al. Phase I/II Study of Intrathecal Administration of Recombinant Human Hepatocyte Growth Factor in Patients with Acute Spinal Cord Injury: A Double-Blind, Randomized Clinical Trial of Safety and Efficacy. J Neurotrauma 2020;37:1752-8. [Crossref] [PubMed]
- Koda M, Hanaoka H, Fujii Y, et al. Randomized trial of granulocyte colony-stimulating factor for spinal cord injury. Brain 2021;144:789-99. [Crossref] [PubMed]
- Nakamura M, Okano H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res 2013;23:70-80. [Crossref] [PubMed]
- Okano H, Sipp D. New trends in cellular therapy. Development 2020;147:dev192567. [Crossref] [PubMed]
- Ruzicka J, Machova-Urdzikova L, Gillick J, et al. A Comparative Study of Three Different Types of Stem Cells for Treatment of Rat Spinal Cord Injury. Cell Transplant 2017;26:585-603. [Crossref] [PubMed]
- Forostyak S, Jendelova P, Sykova E. The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie 2013;95:2257-70. [Crossref] [PubMed]
- Honmou O, Yamashita T, Morita T, et al. Intravenous infusion of auto serum-expanded autologous mesenchymal stem cells in spinal cord injury patients: 13 case series. Clin Neurol Neurosurg 2021;203:106565. [Crossref] [PubMed]
- Jin MC, Medress ZA, Azad TD, et al. Stem cell therapies for acute spinal cord injury in humans: a review. Neurosurg Focus 2019;46:E10. [Crossref] [PubMed]
- Fessler RG, Ehsanian R, Liu CY, et al. A phase 1/2a dose-escalation study of oligodendrocyte progenitor cells in individuals with subacute cervical spinal cord injury. J Neurosurg Spine 2022;37:812-20. [Crossref] [PubMed]
- McKenna SL, Ehsanian R, Liu CY, et al. Ten-year safety of pluripotent stem cell transplantation in acute thoracic spinal cord injury. J Neurosurg Spine 2022; Epub ahead of print. [Crossref] [PubMed]
- Curtis E, Martin JR, Gabel B, et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell 2018;22:941-950.e6. [Crossref] [PubMed]
- Takahashi K, Okita K, Nakagawa M, et al. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc 2007;2:3081-9. [Crossref] [PubMed]
- Seki T, Yuasa S, Oda M, et al. Generation of induced pluripotent stem cells from human terminally differentiated circulating T cells. Cell Stem Cell 2010;7:11-4. [Crossref] [PubMed]
- Umekage M, Sato Y, Takasu N. Overview: an iPS cell stock at CiRA. Inflamm Regen 2019;39:17. [Crossref] [PubMed]
- Hanatani T, Takasu N. CiRA iPSC seed stocks (CiRA's iPSC Stock Project). Stem Cell Res 2020; Epub ahead of print. [Crossref] [PubMed]
- Xu H, Wang B, Ono M, et al. Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility. Cell Stem Cell 2019;24:566-578.e7. [Crossref] [PubMed]
- Nori S, Okada Y, Yasuda A, et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A 2011;108:16825-30. [Crossref] [PubMed]
- Kobayashi Y, Okada Y, Itakura G, et al. Pre-evaluated safe human iPSC-derived neural stem cells promote functional recovery after spinal cord injury in common marmoset without tumorigenicity. PLoS One 2012;7:e52787. [Crossref] [PubMed]
- Fujimoto Y, Abematsu M, Falk A, et al. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cells 2012;30:1163-73. [Crossref] [PubMed]
- Kitagawa T, Nagoshi N, Kamata Y, et al. Modulation by DREADD reveals the therapeutic effect of human iPSC-derived neuronal activity on functional recovery after spinal cord injury. Stem Cell Reports 2022;17:127-42. [Crossref] [PubMed]
- Kawai M, Imaizumi K, Ishikawa M, et al. Long-term selective stimulation of transplanted neural stem/progenitor cells for spinal cord injury improves locomotor function. Cell Rep 2021;37:110019. [Crossref] [PubMed]
- Nichols CD, Roth BL. Engineered G-protein Coupled Receptors are Powerful Tools to Investigate Biological Processes and Behaviors. Front Mol Neurosci 2009;2:16. [Crossref] [PubMed]
- Nori S, Okada Y, Nishimura S, et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Reports 2015;4:360-73. [Crossref] [PubMed]
- Yamanaka S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 2020;27:523-31. [Crossref] [PubMed]
- Sugai K, Fukuzawa R, Shofuda T, et al. Pathological classification of human iPSC-derived neural stem/progenitor cells towards safety assessment of transplantation therapy for CNS diseases. Mol Brain 2016;9:85. [Crossref] [PubMed]
- Iida T, Iwanami A, Sanosaka T, et al. Whole-Genome DNA Methylation Analyses Revealed Epigenetic Instability in Tumorigenic Human iPS Cell-Derived Neural Stem/Progenitor Cells. Stem Cells 2017;35:1316-27. [Crossref] [PubMed]
- Crawford TQ, Roelink H. The notch response inhibitor DAPT enhances neuronal differentiation in embryonic stem cell-derived embryoid bodies independently of sonic hedgehog signaling. Dev Dyn 2007;236:886-92. [Crossref] [PubMed]
- Nelson BR, Hartman BH, Georgi SA, et al. Transient inactivation of Notch signaling synchronizes differentiation of neural progenitor cells. Dev Biol 2007;304:479-98. [Crossref] [PubMed]
- Okubo T, Iwanami A, Kohyama J, et al. Pretreatment with a γ-Secretase Inhibitor Prevents Tumor-like Overgrowth in Human iPSC-Derived Transplants for Spinal Cord Injury. Stem Cell Reports 2016;7:649-63. [Crossref] [PubMed]
- Itakura G, Kawabata S, Ando M, et al. Fail-Safe System against Potential Tumorigenicity after Transplantation of iPSC Derivatives. Stem Cell Reports 2017;8:673-84. [Crossref] [PubMed]
- Kojima K, Miyoshi H, Nagoshi N, et al. Selective Ablation of Tumorigenic Cells Following Human Induced Pluripotent Stem Cell-Derived Neural Stem/Progenitor Cell Transplantation in Spinal Cord Injury. Stem Cells Transl Med 2019;8:260-70. [Crossref] [PubMed]
- Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 2011;365:1673-83. [Crossref] [PubMed]
- Zarogoulidis P, Darwiche K, Sakkas A, et al. Suicide Gene Therapy for Cancer - Current Strategies. J Genet Syndr Gene Ther 2013;4:16849. [PubMed]
- Nori S, Khazaei M, Ahuja CS, et al. Human Oligodendrogenic Neural Progenitor Cells Delivered with Chondroitinase ABC Facilitate Functional Repair of Chronic Spinal Cord Injury. Stem Cell Reports 2018;11:1433-48. [Crossref] [PubMed]
- Zhao Y, Tang F, Xiao Z, et al. Clinical Study of NeuroRegen Scaffold Combined With Human Mesenchymal Stem Cells for the Repair of Chronic Complete Spinal Cord Injury. Cell Transplant 2017;26:891-900. [Crossref] [PubMed]
- Shinozaki M, Iwanami A, Fujiyoshi K, et al. Combined treatment with chondroitinase ABC and treadmill rehabilitation for chronic severe spinal cord injury in adult rats. Neurosci Res 2016;113:37-47. [Crossref] [PubMed]
- Kim KD, Lee KS, Coric D, et al. Acute Implantation of a Bioresorbable Polymer Scaffold in Patients With Complete Thoracic Spinal Cord Injury: 24-Month Follow-up From the INSPIRE Study. Neurosurgery 2022;90:668-75. [Crossref] [PubMed]
- Okubo T, Nagoshi N, Kohyama J, et al. Treatment with a Gamma-Secretase Inhibitor Promotes Functional Recovery in Human iPSC- Derived Transplants for Chronic Spinal Cord Injury. Stem Cell Reports 2018;11:1416-32. [Crossref] [PubMed]
- Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol 2005;486:373-83. [Crossref] [PubMed]
- Khazaei M, Ahuja CS, Fehlings MG. Generation of Oligodendrogenic Spinal Neural Progenitor Cells From Human Induced Pluripotent Stem Cells. Curr Protoc Stem Cell Biol 2017;42:2D.20.1-2D.20.14.
- Nagoshi N, Khazaei M, Ahlfors JE, et al. Human Spinal Oligodendrogenic Neural Progenitor Cells Promote Functional Recovery After Spinal Cord Injury by Axonal Remyelination and Tissue Sparing. Stem Cells Transl Med 2018;7:806-18. [Crossref] [PubMed]
Cite this article as: Inoue M, Yamaguchi R, He CCJ, Ikeda A, Okano H, Kohyama J. Current status and prospects of regenerative medicine for spinal cord injury using human induced pluripotent stem cells: a review. Stem Cell Investig 2023;10:6.


