
The role of macrophages in fracture healing: a narrative review of the recent updates and therapeutic perspectives
Introduction
Bone has a significant regenerative capacity after fracture, being able to completely restore its pre-injury form and function. However, consolidation failures are frequent in patients with long bone fractures resulting from high-energy trauma (traffic accidents, falls from heights, and gunshots) and with fragility of the bone mass, resulting in an inability to heal the fracture (nonunion) (1-4). Treatment options for this problem usually involve surgical removal of the nonunion fibrotic tissue, followed by replacement of the fixation device and grafting at the fracture site with autologous and/or allogeneic trabecular bone containing red marrow (3,5). Nevertheless, if the cause of the nonunion is more a biological, rather than a mechanical impairment, this strategy does not actually fix the source of the problem, and not surprisingly, re-failures are common. The burden is significant for patients who undergo multiple invasive surgeries, prolonged time of chronic pain, physical incapacity, and psychosocial disability. Furthermore, nonunion treatment is expensive, requires permanent medical assistance, multiple hospitalizations, and the use of many orthopedic devices (6,7). Thus, nonunion is already a clinical challenge, and considering the lifestyle in urban centers and the increasing populational aging rates (8), this scenario is expected to worsen, requiring the development of innovative therapeutic strategies to promote bone repair. To this end, a better understanding of the mechanisms that determine the success of fracture healing is a fundamental step.
Long bone fractures treated with fixation strategies that allow a certain degree of movement between the cortical bone ends, such as the gold-standard intramedullary nailing (5,9), heal through callus formation, a process that encompasses three major consecutive phases: the inflammatory reaction, the repair phase, and the final bone remodeling (10-12) (Figure 1). Following the breakage of bone and the rupture of blood vessels, a coagulation cascade is triggered, forming a hematoma that fills the space between bone fragments and connects the bone marrow, the periosteum, the endosteum, and the surrounding muscle. Within this unique microenvironment, immune cells, including neutrophils, macrophages, and platelets, begin an inflammatory reaction with the secretion of cytokines, chemokines and growth factors, such as interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), monocyte chemotactic protein-1 (MCP-1), macrophage colony-stimulating factor (M-CSF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF) (6,10). While this response is amplified within the hematoma, the surrounding periosteum expands, and a new chemotaxis axis mediated by fibroblast growth factors (FGF), bone morphogenetic proteins (BMP), Indian hedgehogs (Ihh), Wnts, placental growth factors (PIGF), and VEGFs activate and recruit endothelial and skeletal stem/progenitor cells, which migrate to specific areas of the fracture to promote revascularization and bone repair (13,14). In mice, at day 7, osteochondroprogenitors expressing runt-related transcription factor 2 (Runx-2) and SRY-Box Transcription Factor 9 (Sox-9) locate at the periphery of the fracture line adjacent to the periosteum (15). On day 14, committed osteoprogenitors expressing Runx2 and Osterix (Osx) are found on both sides of the gap, where new bone formation occurs through intramembranous ossification (15). On the other hand, Sox9+ chondroprogenitors are located exclusively in the central area of the fracture, where they generate chondrocytes and establish a soft callus composed of hyaline cartilage (6,12,13,15). Later, terminal differentiation of chondrocytes into the hypertrophic state stimulates matrix calcification and vascular invasion. Following the path of type H vessels formed by endothelial cells with high expression of Endomucin and CD31 (15), Runx2+ Osx+ osteoprogenitors reach the area of the calcified cartilage matrix and start depositing immature trabecular bone. This hard callus then bridges the old cortical bone fragments, and finally the bone segment is remodeled into the mature lamellar osseous structure by the activity of osteoclasts and osteoblasts, restoring its original shape and mechanical properties (12,16).
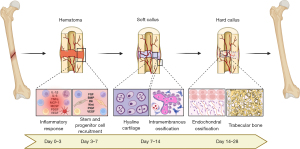
Although this general sequence of fracture healing is well described (6,12), several knowledge gaps still exist in relation to cellular interactions and molecular signaling leading to bone consolidation or nonunion. However, compelling evidence indicates that the initial inflammatory phase is the most critical for the outcome (17-19). At this stage, many types of immune cells are found within the early fracture hematoma, including lymphocytes, which are cells of the adaptive response (20-25). However, the role of macrophages in fracture healing has become a central topic in osteoimmunology research because: (I) macrophages are the main drivers of inflammatory responses in the general process of wound healing, being the source of several chemotactic molecules that activate and recruit both specialized and progenitor/stem cells to engage in tissue repair (26,27); (II) during homeostasis, they are found on the endosteal surface of bones, covering osteoblasts in a canopy-like structure (28) and supporting bone formation in the context of the physiological process of bone remodeling (29-31); (III) macrophages were found to secrete inductive signals that stimulate the differentiation of skeletal (32-35) and endothelial progenitor cells (36,37); (IV) in the context of the fracture healing cascade, macrophages were found within the callus at all stages, in close association with areas of bone formation (21,34,38,39); and (V) their general depletion or the knockdown of receptors that mediate their differentiation, polarization, and/or function resulted in delayed and/or failed bone consolidation in murine fracture healing models (28,34,39-42). Collectively, these findings suggested that the contributions of macrophages to bone repair go far beyond the sole modulation of inflammation, placing these cells as central instructors of the whole healing cascade (43-45). Consequently, modulation of macrophage activity has emerged as a potentially important therapeutic strategy to stimulate or accelerate bone repair. Nevertheless, as macrophages are a heterogenous cell population which can adopt diverse phenotypes and functional profiles (27,46,47), further studies are still needed in order to better depict the specific contributions of the different macrophage subtypes in each stage/event of fracture healing. Therefore, the objective of this review is to address the most recent advances in research on the role of macrophages in the healing of long bone fractures, exploring their relationship with failures in bone consolidation, and the latest perspectives for the development of innovative treatments for delayed and/or failed bone consolidation, based on the modulation of macrophage activity. We present the following article in accordance with the Narrative Review reporting checklist (available at https://sci.amegroups.com/article/view/10.21037/sci-2022-038/rc).
Methods
The literature search was carried out in the PubMed database, using combinations of the keywords “macrophage”, “fracture healing”, “bone regeneration”, and “bone repair”. Articles published within the last years (2017–2022) reporting evidence resulting from in vivo long bone fracture healing experiments were included.
General concepts of macrophages and their role in fracture healing
Macrophages are a phenotypic and functionally diverse set of mononucleated phagocytic cells present in all body tissues that are involved in many physiological and pathological processes, including development, homeostasis, immunological defenses, and repair (48). This macrophage heterogeneity stems both from the origin of the macrophage population (49), as well as from the overall input signaling deriving from the tissue microenvironment at a given context, which makes macrophages polarize between two opposing spectrums: M1, which steers pro-inflammatory responses; and M2, which promotes resolution of inflammation and tissue repair (48). The M1 macrophages are also described as “classically activated macrophages” and phenotypically express high levels of the major histocompatibility complex class II (MHC II), F4/80, CD11b, and CD68 and secrete pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6 (48). On the contrary, M2 macrophages, also known as “alternatively activated macrophages”, express the surface markers F4/80, CD162, and CD206 and secrete cytokines such as arginase-1, IL-4, IL-10, and transforming growth factor beta (TGF-β). These are further subdivided into M2a-M2d, with each subpopulation expressing a specific repertoire of cytokines (48) (Figure 2).
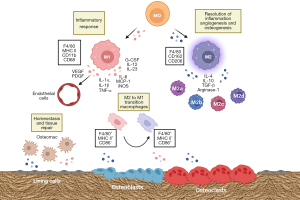
Regarding origin, studies have shown that the population of macrophages that reside within specific tissues [which are collectively called tissue-resident macrophages; TRM (50)] derives from yolk-sac erythromyeloid progenitors and can self-renew, maintaining their pools throughout life (51). Functionally, TRMs were shown to maintain tissue homeostasis and facilitate tissue repair by resolving inflammation. On the other hand, in adult life, macrophages can also originate from blood-circulating monocytes, which derive from bone marrow myeloid progenitors (49). In bones, TRMs are called osteomacs (28,52) (Figure 2). These are found in the endosteum, close to bone lining cells (28,53). On the other hand, recruited macrophages are found to be distributed among the bone marrow and increase in number when a fracture occurs (53).
Studies published in the last five years on the role of macrophages in fracture healing strengthened the idea that what appears to be essential when it comes to a successful consolidation is the right balance between the M1/M2 populations, which have different but complementary roles in the process. The general paradigm of macrophage functional polarization in bone regeneration indicates that M1 macrophages contribute to the establishment of the initial acute inflammatory response within the hematoma, increasing its number early after fracture and secreting pro-inflammatory factors, such as IL-1α, IL-1β, IL-6, MCP-1, granulocyte-colony stimulating factor (G-CSF), IL-12, IL-23, TNF-α, and inducible nitric oxide synthase (iNOS), that will recruit additional immune cells to amplify the response (54). Additionally, these cells clear debris from dying and necrotic cells and produce growth factors, such as VEGF and PDGF, that activate endothelial progenitors that will start the angiogenic process. In fact, McCauley et al. (54) showed that F4/80+/MHC II+/CD86+ M1 macrophages were elevated early after injury in fractured femurs of mice and that this increase consistently progressed over time until day 7, which in mice is marked by the transition from the inflammatory to the tissue neoformation phase. Furthermore, the authors showed that while the M1 macrophage pool increased, M2 macrophages identified by the phenotype F4/80+ MHCII−/CD86−/CD11b− decreased significantly, and this was accompanied by the surge of two additional populations of F4/80+ macrophages with intermediate phenotypes (F4/80+/MHCII+/CD86− and F4/80+/MHCII−/CD86+), which were defined as macrophages transitioning from M2 to M1 (54), thus pointing to the critical role of M1 macrophages for the initiation of the healing cascade.
The importance of M1 macrophages in fracture healing was further highlighted by studies showing that the deletion of this population in mice fracture models resulted in impairment of bone consolidation (55-57). Using the saporin-conjugated Mac-1 antibody to deplete CD11b+ macrophages, Hozain & Cottrell observed a reduction in F4/80+/CD11b+ cells in the callus and a decrease in cartilage and bone volume, trabecular bone volume and thickness, and cortical area in the fractured limb (58). Similarly, Wasnik et al. showed that the administration of 1,25-Dihydroxyvitamin D [1,25(OH)2D] at the fracture site during the early pro-inflammatory stage suppressed M1 and stimulated M2 macrophage differentiation, reducing callus size by approximately 40% on day 14 after fracture and the final bone union rate by 65%. Importantly, these effects were not observed when 1,25(OH)2D treatment was performed after the inflammatory phase (55), which agrees with the notion that the role of M1 macrophages is preponderant at the inflammatory stage of fracture healing, which then subsides with the transition to the tissue neoformation stage, when M2 macrophages dominate and are believed to instruct the mechanisms of angiogenesis and osteogenesis (56). In this regard, using the unicortical drill-hole fracture model in mice, Olmsted-Davis et al. identified a transient population of macrophages that express the beta-3 adrenergic receptor (ADRb3) within the callus, which peaked approximately 4 days after the injury. The authors verified, through immunophenotypic analysis, that these cells were more polarized toward the M2 spectrum, acting to regulate oxygen tension and promote angiogenesis (59).
The relevance of the timely progression of the pro- to anti-inflammatory microenvironment for fracture healing was reported in two studies by Zhao et al. (41,42). In the first, the authors showed that the deficiency in the function of macrophage G-protein coupled receptor interacting protein 1 (GIT1) in a tibial monocortical fracture model resulted in persistent and enhanced M1 macrophage infiltration, exacerbation of IL-1β production, and impairment of bone formation through intramembranous ossification (42). On the other hand, using the same mice fracture model, it was verified that the loss of function of macrophage scavenger receptor 1 (MSR1) in macrophages was correlated with a significant increase in M1 macrophages (F4/80+ iNOS+) and a marked decrease in the M2 pool (F4/80+ CD206+) on day 7 after fracture, also resulting in impaired intramembranous ossification (41).
The role of osteomacs, the TRMs of bones, was also investigated. Using the CD169-diphteria toxin receptor (DTR) knock-in model, Batoon et al. evaluated the effects of osteomac depletion on the regeneration of bone injuries produced by the drill-hole method (which heals by intramembranous ossification) and by complete femoral osteotomy (which heals by endochondral ossification) (60). In vehicle-treated CD169-DTR mice, F4/80+ macrophages were abundantly seen in the adjacent bone marrow area, dispersed throughout the site of the injury associated with the granulation tissue, and accumulated in the peripheral injury zone. However, in CD169-DTR mice treated with diphteria toxin and subjected to the drill hole lesion, F4/80+ macrophages were greatly reduced in the adjacent bone marrow, rare within the granulation tissue, and present, but with a reduced frequency in the peripheral injury zone. This observation was correlated with a reduction in bone formation and an increase in fibrotic tissue at the fracture site. In the endochondral fracture healing model, a general decrease in callus size was observed, whose magnitude was correlated with the number of residual F4/80+ macrophages that remained in the fracture site, suggesting that osteomacs are also important for the success of bone consolidation (60).
Relationship of macrophages and fracture impairment in the context of bone diseases
The disturbed frequency of macrophages and/or of their activity has been reported in various skeletal disease conditions and has been further investigated in the last five years. In a mice model of glucocorticoid-induced delayed bone repair, a common condition in fractured patients who use glucocorticoids to treat chronic inflammatory diseases, Okada et al. verified that dexamethasone treatment significantly decreased the number of F4/80+ cells at the femoral fracture site two days after injury, as well as the mRNA levels of M-CSF, MCP-1, IL-1β, and stromal cell-derived factor-1 (SDF-1, also known as CXCL-12), cytokines involved with macrophage activation and differentiation (61).
Similar findings were reported in a study by Chen et al. (62), in a mouse model of postmenopausal osteoporosis, a condition in which delayed fracture healing is also frequently observed. The authors found that both endochondral ossification and callus remodeling were impaired in ovariectomized mice (OVX), which possessed a decreased expression of TNF-α and IL-6 and a lower frequency of M1 and M2 macrophages in the fracture hematoma, respectively, on days 1 and 14 after the fracture (62).
Diabetes is another recognized condition that adversely affects fracture healing, with patients taking twice the time to heal a fracture and at a higher risk of progressing to nonunion. Using a mouse model of streptozotocin-induced diabetes and the drill hole model, Shimoide et al. showed that the number of macrophages at the fracture site was significantly lower on day 2 after fracture and the mRNA levels of M-CSF, iNOS, IL-6 and CD206 were significantly decreased (63).
In skeletal fluorosis, a clinical manifestation caused by the excessive ingestion of fluoride and its incorporation into hydroxyapatite crystals, delayed fracture healing is also reported. In a rat fracture healing model, Du et al. found that the number of CD86+ M1 macrophages increased at the fracture site on day 7 after the fracture, while the number of CD206+ M2 macrophages decreased. At 21 days, the number of M1 macrophages was still high in the fluoride treated group, indicating that fluoride results in prolongation of the pro-inflammatory stage, and therefore inhibition of the tissue neoformation phase (64).
A prolonged initial inflammatory phase is also reported in fracture healing in aged individuals and is considered to be part of the systemic “inflammaging”, that is, the chronic and increased pro-inflammatory status associated with aging. Clark et al. showed, through bulk mRNA sequencing, that macrophages from old mice have a more pronounced M1 gene signature than macrophages from young animals, and that these M1 macrophages persistently accumulate at the fracture site, thus affecting the resolution of the inflammatory response and callus formation (65). Collectively, these studies strengthened the concept of the major influence of the inflammatory phase on the outcome of fracture healing and of macrophages as central players in the regulation of its first initiation and, later, of its resolution, opening new avenues for the development of treatments to stimulate bone repair.
Perspectives for the development of advanced therapies
Due to its preponderant role in fracture healing, several macrophage-targeted therapies have recently been tested in animal models. The newest research efforts include the following strategies: local or systemic administration of molecules (mainly growth factors and inflammatory modulators) and biomaterials intended to regulate macrophage differentiation and polarization; local transplantation of macrophage precursors; and the use of exosomes to deliver signaling molecules that influence macrophage activities, such as miRNAs (Figure 3).
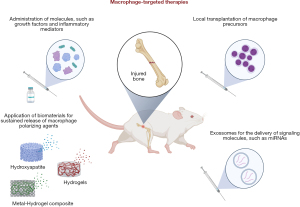
Batoon et al. investigated the effects of intermittent systemic administration of a chimeric M-CSF-1 molecule, which was developed to have an extended circulating half-life compared to native M-CSF-1, in healthy and osteoporotic femur diaphyseal fractures (66). The authors reported an improvement in callus development in healthy mice, which was correlated with an increase in the number of macrophages at the fracture site (66). These findings agreed with the report by Starlinger et al., who showed that systemic application of M-CSF in fractured mice resulted in larger calluses with increased trabecular thickness (67). Most importantly, Batoon et al. showed that M-CSF-1 treatment was also beneficial for the treatment of osteoporotic fractures, in which bone volume within the callus and cortical bridging were increased significantly, culminating in improved fracture biomechanical strength (66).
Similar results were observed in the study by Huang et al., that investigated the impact of Maresin 1 (macrophage mediator in resolving inflammation, MaR1), a macrophage secreted molecule that signals both in autocrine and paracrine ways to decrease macrophage-associated inflammation, for the treatment of tibial fractures in aged mice (68). Upon systemic administration of MaR1, the authors verified that the fracture calluses of aged mice had increased bone volume and better structural stiffness, consistent with overall improved healing. These findings were correlated with a lower percentage of pro-inflammatory macrophages within the fracture callus and a decreased plasmatic level of inflammatory cytokines. In vitro, MaR1 treatment induced the expression of anti-inflammatory markers in macrophages, indicating its potential to act as a polarizing agent, shifting macrophage fate toward an anti-inflammatory phenotype, and contributing to the resolution of inflammation, which is naturally amplified in the elderly (68). Another study, published by Clark et al., showed beneficial effects on the healing of fractures in old mice after the use of a pharmacological agent that antagonizes the M-CSF-1 receptor, thus inhibiting monocyte to macrophage differentiation (65).
Modification of biomaterials as a strategy to immunomodulate the fracture microenvironment has also been recently explored. Applying a biomimetic polysaccharide hydrogel-metal scaffold composite loaded with IL-4 and BMP-2 within femoral defects in rats, Wang et al. observed an increase in the number of M2 macrophages and in the proliferation and differentiation of skeletal progenitors into osteoblasts, which significantly improved bone regeneration (69). In another study, Xu et al. (70) developed an intramedullary nail composed of a copper-containing stainless steel as a strategy to deliver copper ions at the injury site, as this ion had been previously shown to considerably induce M2 macrophage polarization in vitro (71). Using the drill-hole injury model in the tibia of mice, the authors reported an accelerated formation of new cortical bone in the animals that received the copper-enriched intramedullary nail. When the callus was evaluated, it was observed an increased infiltration of CD206+ M2a macrophages, which located close to the newly formed type-H vessels and around the surface of the neoformed osseous tissue (70).
The strategy based on local transplantation of macrophage precursors as an attempt to correct the imbalance between the M1/M2 macrophage pools at the fracture site of aged rats was explored in the study by Löffler et al. (72). The authors reported a partial rescue of bone regeneration after 6 weeks of injury, with increased bone deposition, reduced areas of fibrosis, and improved neovascularization within the calluses, presumably through induction of M2 macrophage differentiation (72). Vi et al. also investigated the possibility of modulating macrophage content within fractured bones through bone marrow transplantation and parabiosis strategies (73). In the study, when old animals received F4/80+ macrophages from young animals, their fracture calluses showed increased bone formation relative to animals that received old F4/80+ cells, indicating the potential of young macrophages to rejuvenate the repair process in aged animals. Among the factors produced by young macrophage cells that could be associated with this rejuvenation effect, the authors highlighted the role of LRP-1 (low-density lipoprotein receptor-related protein 1), which is involved in osteogenic differentiation of skeletal progenitors (73).
At the frontier of knowledge are the strategies that envision the use of exosomes, small vesicles secreted into the circulation by diverse cell types that contain various signaling molecules that once internalized by neighboring or distal cells, can alter the recipient function. Using a femur diaphyseal mice fracture model, Xiong et al. showed that the injection of M2 macrophage-derived exosomes, containing the microRNA miR-5106, at the injury site resulted in calluses with increased bone volume, reduced cartilage area and smaller fracture gaps, indicative of an accelerated fracture healing process (74). In vitro data showed that miR-5106 was able to stimulate osteogenic differentiation of skeletal progenitors, suggesting that the improvements in fracture healing observed in vivo may be related to the stimulation of skeletal cells (74). In a study by Zhang et al., it was verified that the injection of macrophage-derived exosomes isolated from diabetic mice at the fracture site of healthy mice resulted in the development of calluses with significantly lower bone volume and larger fracture gaps (75). The authors identified the microRNA miR-144-5p as responsible for the observed impairments in fracture healing, possibly acting by suppressing osteogenic differentiation of skeletal progenitors (75). As the signaling activities of microRNAs can be molecularly targeted with antagonists, these findings point to a promising new avenue of research that can be further explored to develop innovative therapies to improve fracture healing.
Conclusions
Research on fracture healing has significantly advanced the knowledge about the central cellular and molecular mechanisms that drive bone regeneration and lead to a successful clinical outcome, which ultimately is patient rehabilitation. While the first studies in the field focused on depicting the role of skeletal stem/progenitor cells in the process and how these could be explored to devise therapeutic strategies to promote bone regeneration (13,76-80), we now observe an important paradigm shift, with studies now focusing on the fact that fracture healing is a complex event, influenced by many factors beyond stem/progenitor cells, and that these factors deserve better attention. The role of macrophages in fracture healing has long been recognized (34,39,40,43,45,81-83), and recent literature consolidates this notion, opening promising new avenues for the development of advanced and innovative therapies based on targeted macrophage activities. However, considering that macrophages are highly heterogeneous and their activities must be precisely in concert with the spatio-temporal context of the fracture healing process, more research is still warranted to better understand their diversity (48,84), their specific role in each step of fracture healing, and to decipher the key molecular mechanisms involved in their in vivo crosstalk with other microenvironmental cells, especially with endothelial and skeletal stem/progenitor cells. Although many studies in the literature bring evidence of putative signaling pathways involved in the communication between macrophages and endothelial and skeletal cells, the majority come from in vitro studies, which do not fully recapitulate the complexity of the fracture healing microenvironment. Nevertheless, with the recent advancements in molecular biology tools, such as the diverse omics performed at the single cell level and next-generation sequencing, novel possibilities of investigation are open, increasing the possibility of effectively answering these questions and translating new and effective therapies for those who suffer with incapacitating bone fractures.
Acknowledgments
Funding: This work was supported by the grant E-26/201.403/2022 (to DCB) from the Carlos Chagas Filho Foundation for Research Support in the State of Rio de Janeiro (FAPERJ).
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://sci.amegroups.com/article/view/10.21037/sci-2022-038/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://sci.amegroups.com/article/view/10.21037/sci-2022-038/coif). DCB reports grant E-26/201.403/2022 from the Carlos Chagas Filho Foundation for Research Support in the State of Rio de Janeiro (FAPERJ). The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Mick P, Fischer C. Delayed Fracture Healing. Semin Musculoskelet Radiol 2022;26:329-37. [Crossref] [PubMed]
- Thomas JD, Kehoe JL. Bone Nonunion. In: StatPearls. Treasure Island, FL, USA: StatPearls Publishing, 2020.
- Özkan S, Nolte PA, van den Bekerom MPJ, et al. Diagnosis and management of long-bone nonunions: a nationwide survey. Eur J Trauma Emerg Surg 2019;45:3-11. [Crossref] [PubMed]
- Ding ZC, Lin YK, Gan YK, et al. Molecular pathogenesis of fracture nonunion. J Orthop Translat 2018;14:45-56. [Crossref] [PubMed]
- Giannoudis PV, Krettek C, Lowenberg DW, et al. Fracture Healing Adjuncts-The World's Perspective on What Works. J Orthop Trauma 2018;32:S43-7. [Crossref] [PubMed]
- Kostenuik P, Mirza FM. Fracture healing physiology and the quest for therapies for delayed healing and nonunion. J Orthop Res 2017;35:213-23. [Crossref] [PubMed]
- Kadhim M, Holmes L Jr, Gesheff MG, et al. Treatment Options for Nonunion With Segmental Bone Defects: Systematic Review and Quantitative Evidence Synthesis. J Orthop Trauma 2017;31:111-9. [Crossref] [PubMed]
- Aziziyeh R, Amin M, Habib M, et al. The burden of osteoporosis in four Latin American countries: Brazil, Mexico, Colombia, and Argentina. J Med Econ 2019;22:638-44. [Crossref] [PubMed]
- Bekos A, Sioutis S, Kostroglou A, et al. The history of intramedullary nailing. Int Orthop 2021;45:1355-61. [Crossref] [PubMed]
- Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol 2015;11:45-54. [Crossref] [PubMed]
- Marsell R, Einhorn TA. The biology of fracture healing. Injury 2011;42:551-5. [Crossref] [PubMed]
- Bahney CS, Zondervan RL, Allison P, et al. Cellular biology of fracture healing. J Orthop Res 2019;37:35-50. [Crossref] [PubMed]
- Bragdon BC, Bahney CS. Origin of Reparative Stem Cells in Fracture Healing. Curr Osteoporos Rep 2018;16:490-503. [Crossref] [PubMed]
- Shiu HT, Leung PC, Ko CH. The roles of cellular and molecular components of a hematoma at early stage of bone healing. J Tissue Eng Regen Med 2018;12:e1911-25. [Crossref] [PubMed]
- Stefanowski J, Lang A, Rauch A, et al. Spatial Distribution of Macrophages During Callus Formation and Maturation Reveals Close Crosstalk Between Macrophages and Newly Forming Vessels. Front Immunol 2019;10:2588. [Crossref] [PubMed]
- Oryan A, Monazzah S, Bigham-Sadegh A. Bone injury and fracture healing biology. Biomed Environ Sci 2015;28:57-71. [PubMed]
- Schmidt-Bleek K, Kwee BJ, Mooney DJ, et al. Boon and Bane of Inflammation in Bone Tissue Regeneration and Its Link with Angiogenesis. Tissue Eng Part B Rev 2015;21:354-64. [Crossref] [PubMed]
- Kolar P, Schmidt-Bleek K, Schell H, et al. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev 2010;16:427-34. [Crossref] [PubMed]
- Schmidt-Bleek K, Schell H, Schulz N, et al. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res 2012;347:567-73. [Crossref] [PubMed]
- Baht GS, Vi L, Alman BA. The Role of the Immune Cells in Fracture Healing. Curr Osteoporos Rep 2018;16:138-45. [Crossref] [PubMed]
- Schmidt-Bleek K, Schell H, Kolar P, et al. Cellular composition of the initial fracture hematoma compared to a muscle hematoma: a study in sheep. J Orthop Res 2009;27:1147-51. [Crossref] [PubMed]
- Reinke S, Geissler S, Taylor WR, et al. Terminally differentiated CD8(+) T cells negatively affect bone regeneration in humans. Sci Transl Med 2013;5:177ra36. [Crossref] [PubMed]
- Lopez EM, Leclerc K, Ramsukh M, et al. Modulating the systemic and local adaptive immune response after fracture improves bone regeneration during aging. Bone 2022;157:116324. [Crossref] [PubMed]
- Toben D, Schroeder I, El Khassawna T, et al. Fracture healing is accelerated in the absence of the adaptive immune system. J Bone Miner Res 2011;26:113-24. [Crossref] [PubMed]
- Könnecke I, Serra A, El Khassawna T, et al. T and B cells participate in bone repair by infiltrating the fracture callus in a two-wave fashion. Bone 2014;64:155-65. [Crossref] [PubMed]
- Vannella KM, Wynn TA. Mechanisms of Organ Injury and Repair by Macrophages. Annu Rev Physiol 2017;79:593-617. [Crossref] [PubMed]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008;8:958-69. [Crossref] [PubMed]
- Chang MK, Raggatt LJ, Alexander KA, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol 2008;181:1232-44. [Crossref] [PubMed]
- Cho SW, Soki FN, Koh AJ, et al. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proc Natl Acad Sci U S A 2014;111:1545-50. [Crossref] [PubMed]
- Pettit AR, Chang MK, Hume DA, et al. Osteal macrophages: a new twist on coupling during bone dynamics. Bone 2008;43:976-82. [Crossref] [PubMed]
- Cho SW. Role of osteal macrophages in bone metabolism. J Pathol Transl Med 2015;49:102-4. [Crossref] [PubMed]
- Champagne CM, Takebe J, Offenbacher S, et al. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone 2002;30:26-31. [Crossref] [PubMed]
- Guihard P, Danger Y, Brounais B, et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 2012;30:762-72. [Crossref] [PubMed]
- Vi L, Baht GS, Whetstone H, et al. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res 2015;30:1090-102. [Crossref] [PubMed]
- Gong L, Zhao Y, Zhang Y, et al. The Macrophage Polarization Regulates MSC Osteoblast Differentiation in vitro. Ann Clin Lab Sci 2016;46:65-71. [PubMed]
- Fantin A, Vieira JM, Gestri G, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010;116:829-40. [Crossref] [PubMed]
- Kohara Y, Kitazawa R, Haraguchi R, et al. Macrophages are requisite for angiogenesis of type H vessels during bone regeneration in mice. Bone 2022;154:116200. [Crossref] [PubMed]
- Alexander KA, Raggatt LJ, Millard S, et al. Resting and injury-induced inflamed periosteum contain multiple macrophage subsets that are located at sites of bone growth and regeneration. Immunol Cell Biol 2017;95:7-16. [Crossref] [PubMed]
- Raggatt LJ, Wullschleger ME, Alexander KA, et al. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol 2014;184:3192-204. [Crossref] [PubMed]
- Grundnes O, Reikeraas O. Effects of macrophage activation on bone healing. J Orthop Sci 2000;5:243-7. [Crossref] [PubMed]
- Zhao SJ, Kong FQ, Jie J, et al. Macrophage MSR1 promotes BMSC osteogenic differentiation and M2-like polarization by activating PI3K/AKT/GSK3β/β-catenin pathway. Theranostics 2020;10:17-35. [Crossref] [PubMed]
- Zhao SJ, Liu H, Chen J, et al. Macrophage GIT1 Contributes to Bone Regeneration by Regulating Inflammatory Responses in an ERK/NRF2-Dependent Way. J Bone Miner Res 2020;35:2015-31. [Crossref] [PubMed]
- Wu AC, Raggatt LJ, Alexander KA, et al. Unraveling macrophage contributions to bone repair. Bonekey Rep 2013;2:373. [Crossref] [PubMed]
- Horwood NJ. Macrophage Polarization and Bone Formation: A review. Clin Rev Allergy Immunol 2016;51:79-86. [Crossref] [PubMed]
- Sinder BP, Pettit AR, McCauley LK. Macrophages: Their Emerging Roles in Bone. J Bone Miner Res 2015;30:2140-9. [Crossref] [PubMed]
- Davies LC, Jenkins SJ, Allen JE, et al. Tissue-resident macrophages. Nat Immunol 2013;14:986-95. [Crossref] [PubMed]
- Gordon S, Plüddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev 2014;262:36-55. [Crossref] [PubMed]
- Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol 2020;15:123-47. [Crossref] [PubMed]
- Elhag S, Stremmel C, Zehrer A, et al. Differences in Cell-Intrinsic Inflammatory Programs of Yolk Sac and Bone Marrow Macrophages. Cells 2021;10:3564. [Crossref] [PubMed]
- Nobs SP, Kopf M. Tissue-resident macrophages: guardians of organ homeostasis. Trends Immunol 2021;42:495-507. [Crossref] [PubMed]
- Bian Z, Gong Y, Huang T, et al. Deciphering human macrophage development at single-cell resolution. Nature 2020;582:571-6. [Crossref] [PubMed]
- Batoon L, Millard SM, Raggatt LJ, et al. Osteomacs and Bone Regeneration. Curr Osteoporos Rep 2017;15:385-95. [Crossref] [PubMed]
- Mohamad SF, Gunawan A, Blosser R, et al. Neonatal Osteomacs and Bone Marrow Macrophages Differ in Phenotypic Marker Expression and Function. J Bone Miner Res 2021;36:1580-93. [Crossref] [PubMed]
- McCauley J, Bitsaktsis C, Cottrell J. Macrophage subtype and cytokine expression characterization during the acute inflammatory phase of mouse bone fracture repair. J Orthop Res 2020;38:1693-702. [Crossref] [PubMed]
- Wasnik S, Rundle CH, Baylink DJ, et al. 1,25-Dihydroxyvitamin D suppresses M1 macrophages and promotes M2 differentiation at bone injury sites. JCI Insight 2018;3:e98773. [Crossref] [PubMed]
- Schlundt C, El Khassawna T, Serra A, et al. Macrophages in bone fracture healing: Their essential role in endochondral ossification. Bone 2018;106:78-89. [Crossref] [PubMed]
- Sandberg OH, Tätting L, Bernhardsson ME, et al. Temporal role of macrophages in cancellous bone healing. Bone 2017;101:129-33. [Crossref] [PubMed]
- Hozain S, Cottrell J. CDllb+ targeted depletion of macrophages negatively affects bone fracture healing. Bone 2020;138:115479. [Crossref] [PubMed]
- Olmsted-Davis E, Mejia J, Salisbury E, et al. A Population of M2 Macrophages Associated With Bone Formation. Front Immunol 2021;12:686769. [Crossref] [PubMed]
- Batoon L, Millard SM, Wullschleger ME, et al. CD169(+) macrophages are critical for osteoblast maintenance and promote intramembranous and endochondral ossification during bone repair. Biomaterials 2019;196:51-66. [Crossref] [PubMed]
- Okada K, Kawao N, Nakai D, et al. Role of Macrophages and Plasminogen Activator Inhibitor-1 in Delayed Bone Repair Induced by Glucocorticoids in Mice. Int J Mol Sci 2022;23:478. [Crossref] [PubMed]
- Chen L, Cheng S, Sun K, et al. Changes in macrophage and inflammatory cytokine expressions during fracture healing in an ovariectomized mice model. BMC Musculoskelet Disord 2021;22:494. [Crossref] [PubMed]
- Shimoide T, Kawao N, Tamura Y, et al. Role of Macrophages and Plasminogen Activator Inhibitor-1 in Delayed Bone Repair in Diabetic Female Mice. Endocrinology 2018;159:1875-85. [Crossref] [PubMed]
- Du C, Xiao P, Gao S, et al. High Fluoride Ingestion Impairs Bone Fracture Healing by Attenuating M2 Macrophage Differentiation. Front Bioeng Biotechnol 2022;10:791433. [Crossref] [PubMed]
- Clark D, Brazina S, Yang F, et al. Age-related changes to macrophages are detrimental to fracture healing in mice. Aging Cell 2020;19:e13112. [Crossref] [PubMed]
- Batoon L, Millard SM, Raggatt LJ, et al. Treatment with a long-acting chimeric CSF1 molecule enhances fracture healing of healthy and osteoporotic bones. Biomaterials 2021;275:120936. [Crossref] [PubMed]
- Starlinger J, Sarahrudi K, Kecht M, et al. The influence of M-CSF on fracture healing in a mouse model. Sci Rep 2021;11:22326. [Crossref] [PubMed]
- Huang R, Vi L, Zong X, et al. Maresin 1 resolves aged-associated macrophage inflammation to improve bone regeneration. FASEB J 2020;34:13521-32. [Crossref] [PubMed]
- Wang Y, Feng Z, Liu X, et al. Titanium alloy composited with dual-cytokine releasing polysaccharide hydrogel to enhance osseointegration via osteogenic and macrophage polarization signaling pathways. Regen Biomater 2022;9:rbac003. [Crossref] [PubMed]
- Xu D, Qian J, Guan X, et al. Copper-Containing Alloy as Immunoregulatory Material in Bone Regeneration via Mitochondrial Oxidative Stress. Front Bioeng Biotechnol 2020;8:620629. [Crossref] [PubMed]
- Lin R, Deng C, Li X, et al. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics 2019;9:6300-13. [Crossref] [PubMed]
- Löffler J, Sass FA, Filter S, et al. Compromised Bone Healing in Aged Rats Is Associated With Impaired M2 Macrophage Function. Front Immunol 2019;10:2443. [Crossref] [PubMed]
- Vi L, Baht GS, Soderblom EJ, et al. Macrophage cells secrete factors including LRP1 that orchestrate the rejuvenation of bone repair in mice. Nat Commun 2018;9:5191. [Crossref] [PubMed]
- Xiong Y, Chen L, Yan C, et al. M2 Macrophagy-derived exosomal miRNA-5106 induces bone mesenchymal stem cells towards osteoblastic fate by targeting salt-inducible kinase 2 and 3. J Nanobiotechnology 2020;18:66. [Crossref] [PubMed]
- Zhang D, Wu Y, Li Z, et al. MiR-144-5p, an exosomal miRNA from bone marrow-derived macrophage in type 2 diabetes, impairs bone fracture healing via targeting Smad1. J Nanobiotechnology 2021;19:226. [Crossref] [PubMed]
- Serowoky MA, Arata CE, Crump JG, et al. Skeletal stem cells: insights into maintaining and regenerating the skeleton. Development 2020;147:dev179325. [Crossref] [PubMed]
- Matsushita Y, Ono W, Ono N. Skeletal Stem Cells for Bone Development and Repair: Diversity Matters. Curr Osteoporos Rep 2020;18:189-98. [Crossref] [PubMed]
- Sanghani-Kerai A, McCreary D, Lancashire H, et al. Stem Cell Interventions for Bone Healing: Fractures and Osteoporosis. Curr Stem Cell Res Ther 2018;13:369-77. [Crossref] [PubMed]
- Mousaei Ghasroldasht M, Matin MM, Kazemi Mehrjerdi H, et al. Application of mesenchymal stem cells to enhance non-union bone fracture healing. J Biomed Mater Res A 2019;107:301-11. [Crossref] [PubMed]
- Zhang J, Chen J. Bone Tissue Regeneration - Application of Mesenchymal Stem Cells and Cellular and Molecular Mechanisms. Curr Stem Cell Res Ther 2017;12:357-64. [Crossref] [PubMed]
- Alexander KA, Chang MK, Maylin ER, et al. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res 2011;26:1517-32. [Crossref] [PubMed]
- Kobayashi T, Onodera S, Kondo E, et al. Impaired fracture healing in macrophage migration inhibitory factor-deficient mice. Osteoporos Int 2011;22:1955-65. [Crossref] [PubMed]
- Slade Shantz JA, Yu YY, Andres W, et al. Modulation of macrophage activity during fracture repair has differential effects in young adult and elderly mice. J Orthop Trauma 2014;28:S10-4. [Crossref] [PubMed]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep 2014;6:13. [Crossref] [PubMed]
Cite this article as: Frade BB, Dias RB, Gemini Piperni S, Bonfim DC. The role of macrophages in fracture healing: a narrative review of the recent updates and therapeutic perspectives. Stem Cell Investig 2023;10:4.


