
A selective type of autophagy to maintain glioma stem cell activity
Glioblastoma multiforme (GBM) is the most aggressive and abundance primary brain tumor; presenting, unfortunately, very low chances of recovery; among all the cancer types, it is one of those specially complicated in terms of treatment (1). About three in 100,000 people develop the disease per year; the average age at diagnosis is sixty-four; and it presents an estimate patient survival lower than fifteen months (The 2021 WHO Classification). A relevant number of studies postulate that cancer progression in GBM is the result of a complicated sequence of events that implicates many specific cell types, including i.e., neural stem cells, oligodendrocyte progenitor cells and astrocytes (1). Highly proliferative GBM cancer cells present, together with altered DNA, an important number of folding impaired proteins leading to a remarkably damaged protein homeostasis. Cellular activities such as autophagy, similar to other proteolytic pathways, are highlighted for its preventive potential maintaining proteostasis in GBM cells (1).
Proteostasis is the cellular maintenance of proteome, through controlled regulation of processes involving protein translation, folding, trafficking and degradation. Failure of proteostasis can lead to many different disorders such as cancer, among others (2). Out of the different pathways involved in the control of protein balance, deregulation of autophagy it has been described to be implicated in tumor development (2).
There are three main types of autophagy described in mammals: macroautophagy, microautophagy and chaperone-mediated autophagy (CMA) (2). CMA is, among the described autophagy pathways in mammalian cells, one of those mechanisms with higher levels of selectivity for the degradation of cytosolic proteins in lysosomes (2). It is specialized in targeting specific individual cytosolic proteins that contain a consensus aminoacidic sequence, which lead them to the lysosome-associated membrane protein type 2A (LAMP2A) (2). The critical molecular component of CMA functionality is this specific receptor located at the lysosomal membrane, LAMP2A, a splice variant of the LAMP2 gene. In the CMA process, the protein that is targeted will be drive dock into the LAMP2A located in the membrane of the lysosomes, where dedicated machinery will contribute to internalizing the substrate with the participation of different chaperones and co-chaperones—outside and within the lysosome lumen, to later on be degraded (2). This selective activity is greatly modulated based on surrounding cell types as well as on the cellular signals received, which regulates the amount of functional activity in the cell (3,4).
CMA recognition and degradation of individual proteins facilitates fine tuning of the cellular proteome and replacement of already damaged proteins. Capabilities such as the ones detailed allow CMA to contribute to the cellular processes that include: stress adaptation, quality control maintenance and precise modulation of specific pathways such as metabolism control of the cell, differentiation and reprogramming (2). So, in physiological conditions, CMA activity has the capability of protecting cells by the preservation of cellular integrity—also against malignant transformation. However, once cellular damage reaches a limit where this pathway gets dysregulated, CMA is able to facilitate cells—and that includes now tumorigenic transformed cells, survival and growth—and so contributing to the tumor progression (5,6). Consequently, understanding the basis of this dual role for CMA between physiological/anti-oncogenic and physiopathological/pro-oncogenic becomes essential; and acquires even a higher level of relevance in the case of very aggressive tumors, as the one debated here: GBM.
Different projects have been determined the elevated levels of CMA activity—constitutively, in a relevant number of cancer cell types; such upregulation of the pathway is needed to maintain energetic adaptations of tumorigenic cells i.e., the Warburg effect (7). It has been more recently described that samples from patients treated with temozolomide (TMZ) promote expression of LAMP2A-compared with those samples collected at pretreated stages (8). Additionally, Valdor et al. demonstrated how the increased activity of CMA through the induction of LAMP2A expression in pericytes by GBM, enhanced progression of the tumor (9). But there was no other evidence relating CMA to glioblastoma stem cell population (GSCs); which is the focus of the paper we highlight here.
Interestingly, Auzmendi-Iriarte et al. (10) showed a constitutional activity for CMA in the maintenance of GSC by regulating a variety of processes and pathways (Figure 1). In addition to validate and extend the results in which LAMP2A increased were detected, higher amounts of LAMP2 have been linked to deficient overall survival in different (wide and global) GBM cohorts managed by the authors. They unveiled how the lack of LAMP2A knockdown make GSCs responsive to treatment with TMZ, while patients presenting GBM -not responding to TMZ, report increased levels of LAMP2 when compared with those responding to the treatment. The study also includes data of a CMA reporter (11) that confirmed functional upregulation of CMA (10).
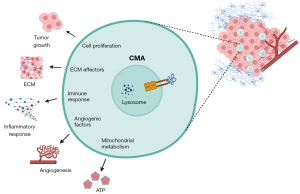
CMA could control GSC activity by multiple processes as shown by proteomic and transcriptomic analysis in the paper (10). The modulation occurs in relation to different regulatory components—also to functional protein groups already described as substrates for this pathway. Those include: extra cellular matrix effectors, pathways related mitochondrial metabolism and the immune system, and p53 or PI3K-AKT pathways (10).
Energy homeostasis of the cells was reported during the last few years as intimately linked with the activity of CMA (3,4). Here, the authors also revealed that downregulation of LAMP2A decreases mitochondrial activity in GSCs, responsible of a diminished ATP production. GSCs knockdown for LAMP2A shown—together with mitochondrial dysfunction, glycolysis upregulation; and so, suggests a potential compensatory mechanism for the ATP acquirement. Contrary to other cancer cases, in GSCs, the changes described in glycolysis looks to be reactive to the mitochondrial function—which is shown as the primary defect (10).
The massive infiltrate capability of GBM tumor cells is also a well stablish hallmark, and key remodeling component of the extracellular matrix (ECM). The results show the impact of decreased expression of LAMP2A downregulating relevant molecules in the interaction of GSCs with ECM, and so driving to the understanding on how CMA regulates the fulfilled ECM and its interplay with GSC (10).
Additionally, the proteomics analysis highlights an IFN signaling enhancement, as well as a decreased of MHC class II antigen-presentation—what together with proinflammatory cytokines, suggest the implication of this selective autophagy pathway in the immune effect in GBM mediated by the STAT components (10). The specific critical cytokines and factors are still pending of further validation; as it is also pending to check if the effect is specific of GSCs.
The most diminished factors associated with GBM progression include insulin growth factor binding proteins (IGFBP), ILs, and angiogenic factors. Their results indicated that elevated CMA activity in GSCs may be involved in the angiogenic growth factors release, to stimulate their interplay to the tumor-associated pericytes, and so probably participating into survival of the tumor (10,12). Altogether, at the molecular level, these data sustained that LAMP2A modulates a variety of regulatory pathways, that includes, among others: activity of the mitochondrial and immune system, and ECM interplay in GSC.
Overall, this excellent research work illustrates that -the expression of LAMP2A and the enriched CMA activity in GSC, is associated with poor patient survival, and it is necessary for the maintenance of GSC tumorigenic activities.
Acknowledgments
We apologize to those esteemed colleagues whose work may have been omitted from this review due to space limitation.
Funding: Work in EA laboratory was supported by the National Institutes of Health [DK124308, P30DK041296 (P&F) and P30AG038072 (P&F)].
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Stem Cell Investigation. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://sci.amegroups.com/article/view/10.21037/sci-2022-047/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Salinas MD, Valdor R. Chaperone-Mediated Autophagy in Pericytes: A Key Target for the Development of New Treatments against Glioblastoma Progression. Int J Mol Sci 2022;23:8886. [Crossref] [PubMed]
- Arias E, Cuervo AM. Chaperone-mediated autophagy in protein quality control. Curr Opin Cell Biol 2011;23:184-9. [Crossref] [PubMed]
- Arias E, Koga H, Diaz A, et al. Lysosomal mTORC2/PHLPP1/Akt Regulate Chaperone-Mediated Autophagy. Mol Cell 2015;59:270-84. [Crossref] [PubMed]
- Arias E. Lysosomal mTORC2/PHLPP1/Akt axis: a new point of control of chaperone-mediated autophagy. Oncotarget 2015;6:35147-8. [Crossref] [PubMed]
- Arias E, Cuervo AM. Pros and Cons of Chaperone-Mediated Autophagy in Cancer Biology. Trends Endocrinol Metab 2020;31:53-66. [Crossref] [PubMed]
- Gómez-Sintes R, Arias E. Chaperone-mediated autophagy and disease: Implications for cancer and neurodegeneration. Mol Aspects Med 2021;82:101025. [Crossref] [PubMed]
- Kon M, Cuervo AM. Chaperone-mediated autophagy in health and disease. FEBS Lett 2010;584:1399-404. [Crossref] [PubMed]
- Natsumeda M, Aoki H, Miyahara H, et al. Induction of autophagy in temozolomide treated malignant gliomas. Neuropathology 2011;31:486-93. [Crossref] [PubMed]
- Valdor R, García-Bernal D, Riquelme D, et al. Glioblastoma ablates pericytes antitumor immune function through aberrant up-regulation of chaperone-mediated autophagy. Proc Natl Acad Sci U S A 2019;116:20655-65. [Crossref] [PubMed]
- Auzmendi-Iriarte J, Otaegi-Ugartemendia M, Carrasco-Garcia E, et al. Chaperone-Mediated Autophagy Controls Proteomic and Transcriptomic Pathways to Maintain Glioma Stem Cell Activity. Cancer Res 2022;82:1283-97. [Crossref] [PubMed]
- Dong S, Aguirre-Hernandez C, Scrivo A, et al. Monitoring spatiotemporal changes in chaperone-mediated autophagy in vivo. Nat Commun 2020;11:645. [Crossref] [PubMed]
- Auzmendi-Iriarte J, Matheu A. Intrinsic role of chaperone-mediated autophagy in cancer stem cell maintenance. Autophagy 2022;18:3035-6. [Crossref] [PubMed]
Cite this article as: Patel K, Arias E. A selective type of autophagy to maintain glioma stem cell activity. Stem Cell Investig 2023;10:1.


