Acute promyelocytic leukemia co-existing with JAK2 V617F positive myeloproliferative neoplasm: a case report
Introduction
The Janus-associated kinase 2 (JAK2) V617F mutation is detected in over 95% of patients with polycythemia vera (PV) and in about 50% of cases of essential thrombocythemia (ET) and primary myelofibrosis (PMF) (1-3). Transformation of myeloproliferative neoplasms (MPN) into acute myelogenous leukemia (AML) is a well-studied and reported phenomenon (2,4,5). Both transformation of ET to AML (6,7), as well as a JAK2 V617F mutation in de novo AML are very rare (8). Amongst all subtypes of AML originating from MPN, acute promyelocytic leukemia (APL) is extremely rare. In the English literature there have been only 9 such cases reported to date (1,7,9-14). In the same literature search we have not found any case of APL with concurrent diagnosis of MPN. Herein, we present the case of a young male with new onset APL and JAK2 positive MPN.
Case presentation
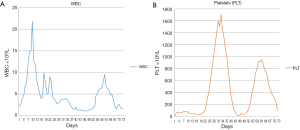
A young male (<40 years old) was referred to our hospital for gingival bleeding, pancytopenia and fever. The patient had previously presented with fever, sore throat, and tonsillar enlargement and had been treated empirically for pharyngitis with antibiotics. He also reported gingival bleeding and several episodes of epistaxis as well as dark stools in the few weeks preceding admission. Physical examination was significant for gingival bleeding and enlarged tonsils. Abdominal ultrasonography revealed splenomegaly with longest diameter of 14.3 cm. A complete blood count on admission was remarkable for a white blood count (WBC) of 2.1×109/L, hemoglobin (Hgb) of 9.8 g/dL, and platelet count of 11×109/L. The peripheral blood smear revealed 10% blasts and 42% promyelocytes. Bone marrow biopsy done on admission showed sheets of immature atypical myeloid cells, comprising more than 90% of the marrow cellularity. Those cells showed moderate to abundant cytoplasm, irregular nuclei (some of which were indented or bilobed), smudged chromatin and prominent nucleoli. Maturing myeloid elements including segmented neutrophils were not seen; megakaryocytes and erythroid precursors were markedly decreased. No reticulin fibrosis was seen. Karyotyping revealed t(15;17)(q24;q21) in all 20 metaphases analyzed. Molecular studies were positive for PML/RARa, FLT3 TKD and JAK2 V617F mutations (Table 1). Additional molecular studies for genes associated with MPN, MDS, and AML were negative (Table 2) (15). The patient was immediately started on all-trans retinoic acid (ATRA). Arsenic trioxide (ATO) was added on day 10 according to the reported regimen for clinically low-risk APL (16). The hospital course was complicated by differentiation syndrome with a high WBC (Figure 1A) and pleural and pericardial effusions for which a short course of dexamethasone was given. Surprisingly, a rapid increase in platelet count was observed during count recovery, with values reaching as high as 1,700×109/L (Figure 1B). Bone marrow biopsy at this point showed increased reticulin fibrosis, a left shift in myeloid lineage cells with dysplastic and an increased number of megakaryocytes. The morphology overall was reported to be consistent with MPN (PMF/ET). A FISH panel for myelodysplasia was negative and a chromosome study revealed a normal karyotype: 46 XY. Molecular studies were negative for the FLT3 TKD, but remained positive for the JAK2 V617F mutation. The PML/RARa was still detectable by PCR post-induction therapy with ATRA and ATO. The patient was started on consolidation chemotherapy with cytarabine and idarubicin for a total of two cycles. He continued ATRA throughout consolidation. The patient became PCR negative for PML/RARa after completion of two cycles of consolidation chemotherapy, however the JAK2 mutation remained positive (Table 1). At that point the patient had a normal WBC but a high platelet count, with platelets as high as 949×109/L. Since the patient presented with leukemia transformation, peak counts of platelets were higher than 1,500×109/L, and bone marrow biopsy revealed dysplastic megakaryocytes as well as reticulin fibrosis, we believe that this patient might more likely have PMF than ET, and had high risk PMF/ET, even though he was younger than 40 (17). Therefore, low dose aspirin (81 mg) and hydroxyurea 1,000 mg daily were given for the PMF/ET. Interferon and ruxolitinib were discussed but clinically impractical at the time. The patient was also placed on maintenance treatment for APL with ATRA every 3 months.

Full table

Full table
Discussion
Both ET and PV may progress to myelofibrosis, and all MPNs may evolve into AML. The most common to transform in this way is PMF, occurring in about 15% of cases (1,18). Such transformation happens even less frequently in patients with ET (6,7). In the majority of PV cases there are clones homozygous for JAK2 V617F mutations, which are rarely found in ET (19,20). JAK2 V617F mutations are very rare in de novo AML (8). Amongst all subtypes of AML originating from MPNs, APL has been reported the least frequently. Only 2 of the early case reports had molecularly documented PML/RARa mutation (7,12) (Table 3). The JAK2 V617F mutation status was unknown in 8 of the cases (1), as they were reported prior to the 2005 discovery of the JAK2 mutation (21,22). The association between APL and MPNs was thought to be due to promyelocytic blastic crisis of MPN, APL secondary to cytoreductive therapy, and de novo APL (7). Braun at al. recently reported a case of APL with PML/RARα and JAK2 V617F mutation (1). The authors postulated that the inflammatory response resulting from the chemokine release (CLL-2 and IL-8) from ATRA-treated APL cells was accentuated by inflammatory downstream signalling from the JAK-2 mutation and that this led to severe differentiation syndrome. JAK-2 inhibition by ruxolitinib in the in vitro studies on NB-4 APL cells treated with ATRA did not affect CLL-2 and IL-8 levels, supporting the importance of JAK2 in downstream activation as previously described (1,2). However, the role of JAK-2 inhibition in the management of differentiation syndrome has not been clinically proven. In the patient presented by Braun et al. the diagnosis of MPN predated the diagnosis of APL. Our report presents for the first time a case of APL diagnosed concurrently with a MPN. It is highly possible that the APL clone remained dominant and masked the phenotype of MPN at the time of diagnosis. The MPN clone became dominant after the APL clone was suppressed. It remains unknown whether the APL clone arose from the MPN clone since we were not able to perform single cell genome analysis prior to the initiation of the APL therapy like in those cases reported in the literature (23-25). Since splenomegaly and FLT3-TKD were also present at the time of diagnosis, we hypothesize that the JAK2 V617F mutated MPN clone (likely PMF) was present first and that additional mutations like FLT3 and PML/RARa took place later and then led to the development of APL (26-32). It is of interest to point out that the case of APL transformed from a known diagnosis of ET with fibrosis also was found to have FLT3-TKD (D835 mutation) in addition to JAK2 V617F mutation (1). These two cases of APL transformation from ET /PMF containing FLT3-TKD mutation make it likely that FLT3-TKD mutation plays a driver role for this transformation process involving PML/RARa.

Full table
Conclusions
This is the first case with molecular data showing co-existence of PML/RARa, FLT3-TKD, and a JAK2 V617F mutation at the time of APL diagnosis. We hypothesize that the JAK2 V617F mutated MPN clone was present first, and that additional mutations like FLT3 and PML/RARa took place later and then led to the development of APL.
Acknowledgements
J Wu is a recipient of the Henan Provincial Grant for Overseas Research for Young Leaders of Medical Technology (No. 2014041). In addition, J Wu also received grant support from the Natural Science Foundation of China (NSFC No. 81201793). The grants supported her research training at the Division of Hematology and Oncology, New York Medical College, USA.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Unfortunately due to special circumstance in this case, consent could not be obtained for publication. The authors ensured that there was no identifiable information in the case (i.e., no race and age were reported). We believe this case has scientific value and is important for clinical literature.
References
- Braun TP, Maxson JE, Agarwal A, et al. Acute promyelocytic leukemia with JAK2 V617F and severe differentiation syndrome. Leuk Res Rep 2014;4:8-11. [Crossref] [PubMed]
- Kralovics R, Passamonti F, Buser AS, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005;352:1779-90. [Crossref] [PubMed]
- Furqan M, Mukhi N, Lee B, et al. Dysregulation of JAK-STAT pathway in hematological malignancies and JAK inhibitors for clinical application. Biomark Res 2013;1:5. [PubMed]
- Björkholm M, Derolf AR, Hultcrantz M, et al. Treatment-related risk factors for transformation to acute myeloid leukemia and myelodysplastic syndromes in myeloproliferative neoplasms. J Clin Oncol 2011;29:2410-5. [Crossref] [PubMed]
- Nagai Y. A case of minor BCR-ABL1 positive acute lymphoblastic leukemia following essential thrombocythemia and originating from a clone distinct from that harboring the JAK2-V617F mutation. Exp Hematol Oncol 2014;3:6. [Crossref] [PubMed]
- Andersson PO, Ridell B, Wadenvik H, et al. Leukemic transformation of essential thrombocythemia without previous cytoreductive treatment. Ann Hematol 2000;79:40-2. [Crossref] [PubMed]
- Sato N, Furukawa T, Masuko M, et al. Acute promyelocytic leukemia developing in untreated essential thrombocythemia. Am J Hematol 2002;71:114-6. [Crossref] [PubMed]
- Theocharides A, Boissinot M, Girodon F, et al. Leukemic blasts in transformed JAK2-V617F-positive myeloproliferative disorders are frequently negative for the JAK2-V617F mutation. Blood 2007;110:375-9. [Crossref] [PubMed]
- Batlle M, Fernández-Avilés F, Ribera JM, et al. Acute promyelocytic leukemia in a patient with idiopathic myelofibrosis. Leukemia 1999;13:492-4. [Crossref] [PubMed]
- Kajiguchi T, Simokawa T, Saito M, et al. Transformation of polycythemia vera to acute promyelocytic leukemia. Int J Hematol 2000;72:520-1. [PubMed]
- Löfvenberg E, Nordenson I, Wahlin A. Cytogenetic abnormalities and leukemic transformation in hydroxyurea-treated patients with Philadelphia chromosome negative chronic myeloproliferative disease. Cancer Genet Cytogenet 1990;49:57-67. [Crossref] [PubMed]
- Mollee PN, Taylor KM, Williams B, et al. Long-term molecular remission in promyelocytic transformation of myeloproliferative disease. Leukemia 1999;13:648-50. [Crossref] [PubMed]
- Ratti M, Ghio R, Casciaro S, et al. Acute promyelocytic leukaemia developing in essential thrombocythaemia: blastic crisis or secondary acute leukaemia? Case report. Haematologica 1984;69:330-5. [PubMed]
- Sessarego M, Defferrari R, Dejana AM, et al. Cytogenetic analysis in essential thrombocythemia at diagnosis and at transformation. A 12-year study. Cancer Genet Cytogenet 1989;43:57-65. [Crossref] [PubMed]
- Li B, Gale RP, Xiao Z. Molecular genetics of chronic neutrophilic leukemia, chronic myelomonocytic leukemia and atypical chronic myeloid leukemia. J Hematol Oncol 2014;7:93. [Crossref] [PubMed]
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med 2013;369:111-21. [Crossref] [PubMed]
- Beer PA, Erber WN, Campbell PJ, et al. How I treat essential thrombocythemia. Blood 2011;117:1472-82. [Crossref] [PubMed]
- Cervantes F. How I treat myelofibrosis. Blood 2014;124:2635-42. [Crossref] [PubMed]
- Vannucchi AM. How I treat polycythemia vera. Blood 2014;124:3212-20. [Crossref] [PubMed]
- Tefferi A, Guglielmelli P, Larson DR, et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood 2014;124:2507-13. [Crossref] [PubMed]
- James C, Ugo V, Casadevall N, et al. A JAK2 mutation in myeloproliferative disorders: pathogenesis and therapeutic and scientific prospects. Trends Mol Med 2005;11:546-54. [Crossref] [PubMed]
- James C, Ugo V, Le Couédic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005;434:1144-8. [Crossref] [PubMed]
- Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 2014;20:1472-8. [Crossref] [PubMed]
- Engle EK, Fisher DA, Miller CA, et al. Clonal evolution revealed by whole genome sequencing in a case of primary myelofibrosis transformed to secondary acute myeloid leukemia. Leukemia 2015;29:869-76. [Crossref] [PubMed]
- Hughes AE, Magrini V, Demeter R, et al. Clonal architecture of secondary acute myeloid leukemia defined by single-cell sequencing. PLoS Genet 2014;10:e1004462. [Crossref] [PubMed]
- Guglielmelli P, Lasho TL, Rotunno G, et al. The number of prognostically detrimental mutations and prognosis in primary myelofibrosis: an international study of 797 patients. Leukemia 2014;28:1804-10. [Crossref] [PubMed]
- Albano F, Anelli L, Zagaria A, et al. SETBP1 and miR_4319 dysregulation in primary myelofibrosis progression to acute myeloid leukemia. J Hematol Oncol 2012;5:48. [Crossref] [PubMed]
- Ferrer-Marín F, Bellosillo B, Martínez-Avilés L, et al. Leukemic transformation driven by an ASXL1 mutation after a JAK2V617F-positive primary myelofibrosis: clonal evolution and hierarchy revealed by next-generation sequencing. J Hematol Oncol 2013;6:68. [Crossref] [PubMed]
- Gelsi-Boyer V, Brecqueville M, Devillier R, et al. Mutations in ASXL1 are associated with poor prognosis across the spectrum of malignant myeloid diseases. J Hematol Oncol 2012;5:12. [Crossref] [PubMed]
- Randhawa J, Ostojic A, Vrhovac R, et al. Splenomegaly in myelofibrosis--new options for therapy and the therapeutic potential of Janus kinase 2 inhibitors. J Hematol Oncol 2012;5:43. [PubMed]
- Tognon R, Gasparotto EP, Neves RP, et al. Deregulation of apoptosis-related genes is associated with PRV1 overexpression and JAK2 V617F allele burden in Essential Thrombocythemia and Myelofibrosis. J Hematol Oncol 2012;5:2. [Crossref] [PubMed]
- Cole CB, Verdoni AM, Ketkar S, et al. PML-RARA requires DNA methyltransferase 3A to initiate acute promyelocytic leukemia. J Clin Invest 2016;126:85-98. [Crossref] [PubMed]
Cite this article as: Mamorska-Dyga A, Wu J, Khattar P, Ronny FM, Islam H, Seiter K, Liu D. Acute promyelocytic leukemia co-existing with JAK2 V617F positive myeloproliferative neoplasm: a case report. Stem Cell Investig 2016;3:8.


