Influence of IL-3 functional fragment on cord blood stem cell ex vivo expansion and differentiation
Introduction
Hematopoietic stem cells (HSCs) exist in bone marrow, mobilized peripheral blood, and fresh umbilical cord blood (UCB). HSCs, which can differentiate into all types of blood cells, play significant roles in building the immune system (1,2). HSC transplantation is widely used to treat blood and immune system diseases. As one of the major resources of HSC, UCB contains large number of HSCs and it is easy to obtain, with no risk of harming the donors. However, the absolute number of HSCs in each UCB sample is still limiting for its clinical applications in adult patients (3-8). To overcome this problem, it is necessary to develop robust systems for ex vivo expansion of HSCs, so that sufficient number of HSCs can be obtained to achieve therapeutic effects.
The human interleukin-3 (hIL-3) is a glycoprotein, which served as a key modulation factor of primitive hematopoietic cell proliferation and differentiation. Previous studies have shown that the combined use of IL-3 and other cytokines, including stem cell factor, thrombopoietin, FLT-3 ligand, and granulocyte colony stimulating factor can effectively stimulate the ex vivo expansion and differentiation for UCB CD34+ cells (9). The CD34 marker is found on the surface of HSCs. Previous studies have established that CD34+ cells are capable of colony formation and proliferation, features that support the designation of CD34 as a marker of stem cells (10). Notably, the mechanisms of IL-3’s action on HSCs, including the locations of functionally important structural domains, are not yet known. A better understanding of the mode of IL-3 action may help to further optimize systems for ex vivo expansion and differentiation for HSCs.
The aim of this study was to obtain novel antibodies that can be used for structural and functional characterization of IL-3. Using a prokaryotic expression system, we obtained recombinant human interleukin-3 (rhIL-3) with biological activities for preparation of monoclonal antibodies (mAb) against rhIL-3. Overlapped peptides of IL-3 were synthesized and each fragment of the synthesized peptides was tested for its enhancement on HSC CD34+ cell expansion and differentiation. Here, we report the production and characterization of new mAb specific for rhIL-3; fragments of IL-3 (peptide 3 and 8) enhances ex vivo HSC CD34+ cell expansion and differentiation. We show that the antibody can neutralize the stimulating effect of IL-3 and the fragment [3] and [8] on ex vivo HSC expansion and differentiation; and we present evidence that the functional fragments of IL-3 for HSC expansion are located from 28 to 49 amino acids, as well as 107 to 127 amino acids in human IL-3 molecule, respectively. Our findings confirmed that the functional peptides promote HSC proliferation and differentiation ex vivo, which will definitely benefit the stem cell research work, since the cost to synthesize peptides is extremely lower than protein expression and purification. Further study will be carried out to identify the in vivo potentials of those minimum epitopes on hematopoietic regeneration and stem cell priming.
Methods
Ethics statement
All research involving animals was conducted according to relevant national and international guidelines. Female BALB/c mice (specific pathogen-free; 8-10 weeks old, weight 18.0–25.6 g), obtained from the Experimental Animal Center of Soochow University (Suzhou, China), were used for mAb production. The experiment protocols were approved by the Institutional Animal Care and Use Committees of Soochow University [IACUC permit number: SYXK(Su) 2012-0045], and were in accordance with the Guidelines for the Care and Use of Laboratory Animals (National Research Council, People’s Republic of China, 2010). We further attest that all efforts were made to ensure minimal suffering.
Antibody production and isotype identification
Purified rhIL-3 with biological activity was obtained from a prokaryotic expression system as described previously (9,11). Briefly, BL21 E. coli transfected with rhIL-3-expressing plasmid was cultured in YT medium, and rhIL-3 expression was induced by the addition of isopropylthio-b-d-galactoside (IPTG). Purified rhIL-3 was obtained after dialysis of inclusion body against a serial of refolding buffers, CM-Sepharose, and Supersex-75 chromatography. Activity of the purified rhIL-3 was confirmed by ex vivo cord blood expansion assays, as described below. Purified rhIL-3 with biological activity was used for mAb production in mice, using standard methods developed in this lab (12). Spleen cells from immunized mice were fused with sp2/0 myeloma; the resulting hybridomas were cultured in HAT medium, and the supernatants of the culture were screened for affinity toward rhIL-3 using ELISA. Positive cultures were then limiting-diluted for isolation of mAb cell lines. The mAbs obtained from the supernatant of individual mAb cell lines were tested on Western blots for specificity. A mouse mAb isotyping reagent kit (Sigma, USA) was used to identify the mAb subtype.
Western blot analysis
Western blot analysis was performed essentially as described (9,13); protein samples were separated on denaturing SDS-polyacrylamide (15%, w/v) gels, before being transferred to polyvinylidene difluoride (PVDF) membranes. Goat-anti-mouse immunoglobulin G (IgG) conjugated with alkaline phosphatase (Biolegend, Canada) was used as secondary antibody, and O-phenylenediamine (Sigma, USA) was used for visualization of detected bands. Prestained protein molecular weight markers (Bio-Rad, USA) were used for size determination. Recombinant human granulocyte colony-stimulating factor, prepared as described (9), was used as a negative control for demonstrating mAb specificity.
Umbilical cord blood (UCB) collection and CD34+ cells isolation
Fresh UCB samples from anonymous, discarded tissue were provided by the Suzhou Municipal Hospital Affiliated Nanjing Medical University (Suzhou, China); the study was approved by the Hospital's Ethics Committee and Research Ethics Advisory Committee. UCB CD34+ cells were isolated from total mononuclear cells (MNC) with the MACS immunomagnetic absorption column separation device and CD34 MicroBead Kit, according to the manufacturer’s instructions (Miltenyi Biotec, Germany). MNC were obtained by density centrifugation, with use of Ficolle-Hypaque Premium (GE healthcare, USA). The purity of CD34+ cells was verified using flow cytometry, with an anti-human CD34 mAb conjugated with phycoerythrin (PE) (Miltenyi Biotec, German) and the model BD FACSVerse flow cytometer (BD, USA).
Inhibitory assay for ex vivo expansion and differentiation of cord blood CD34+ cells with anti-rhIL-3 mAbs
CD34+ cells isolated from an individual UCB sample were divided equally into 27 wells, 3 wells per group, on a 96-well ultra-low attachment microplate with round bottom (Corning, USA). Cells (~7.3×104 in each well) were cultured in 200 μL of STEM PRO®-34SFM medium (with 10% fetal bovine serum, 100 ng/mL Pen Strep, and 2 mM L-glutamine; Gibco, USA), containing 50 ng/mL thrombopoietin and 200 ng/mL rhFLT-3 (Pepro Tech, USA). Additional reagents were added to the following groups: positive control groups, 200 ng/mL reference rhIL-3 (Pepro Tech, USA) or rhIL-3 produced in this laboratory; negative control groups, none or mAb buffer (20 mM Tris-HCl buffer, pH 7.0); and mAb addition groups, 200 ng/mL rhIL-3 (produced in this laboratory) and anti-rhIL-3 mAb added at 0.5, or 2 molar fold to rhIL-3. The mAb or the buffer was pre-incubated with rhIL-3 at 37 °C for 0.5 h before they were added to the assay wells. All groups were cultured for 7 days at 37 °C, under humidified air containing 5% CO2. Culture medium was replenished on day 3.
Abundance of CD34+ cells and total MNCs in the various experimental groups was determined by flow cytometric analysis using anti-human CD34-PE mAb and isotypical IgG1 (Mouse-PE) (RD, USA), respectively. Each sample was incubated with the antibodies at 23 °C for 30 min, in the dark; the cells were then washed and analyzed using a BD FACSVerse flow cytometer.
Overlapped IL-3 peptide fragment synthesis and localization of specific mAbs for synthesized IL-3 fragments by ELISA
A total of 11 peptide fragments were designed and synthesized (Sangon Inc. Shang Hai) to cover the full length of IL-3, with 3-5 amino acids overlapped. They were 1–20; 17–31; 28–49; 46–67; 64–83; 81–94; 91–110; 107–127; 124-138; 135–145; and 142–152. Antigen binding sites or epitopes against anti-rhIL-3 E1 strain and C1 strain were determined by ELISA assay with each individual peptide fragment, using standard protocol. Briefly, 1 μg/100 μL of each peptide was pre-coated to a 96-well-plate, as well as the control wells. RhIL-3 protein was employed here as the positive control. All tests were carried out in triplicates. After blocking step, E1 strain MAb (1:200 dilution), and C1 strain MAb (1:400 dilution) were applied to each well, respectively, and incubated at 37 °C for 1h. Biotin conjugated goat anti-mouse-IgG (Biolegend, San Diego, CA, 1:3,000 dilution), Streptavidin-peroxidase (Sigma-Aldrich, St. Louis, MO, 1:6,000 dilution), and the HRP substrate O-Phenylene Diamine (OPD, Sigma-Aldrich, St. Louis, MO, 40 mg/100 mL) were then incubated sequentially for color development. Optical density (OD) values were read at 490 nm wave length with M3550 microplate reader (Bio-Rad, Hercules, CA).
Test of the functional synthesized fragments for ex vivo expansion and differentiation of cord blood CD34+ cells
CD34+ cells were isolated as described above, and then cultured (7~8×104 in each well of a 24-well-plate) in 1 mL of STEM PRO®-34SFM medium, supplemented with 10% fetal bovine serum, 100 ng/mL Pen/Strep, and 2 mM L-glutamine (Gibco, USA); containing 20 ng/mL thrombopoietin, 100 ng/mL stem cell factor (produced in this lab) and 100 ng/mL rhFLT-3 (Pepro Tech, USA). Additional reagents were added to the following groups: negative control groups without additional reagent added; rhIL3 protein (25 ng/mL) produced in this laboratory were served as positive control; peptide 3, peptide 8 and another random peptide (25 ng/mL, each) were also tested side by side; mAb addition groups, 25 ng/mL positive peptides or random negative peptide along with anti-rhIL-3 mAb added at 0.5 and 2 molar fold to the rhIL3 or peptides. The mAb or the buffer was pre-incubated with the peptides (or rhIL-3) at 37 °C for 0.5 h before they were added to the assay wells. All groups were cultured for 7 days in the incubator with humidified air containing 5% CO2. Culture medium was replenished on day 3. The number of CD34+ cells and total nucleated cells in the various treatment groups were detected by FACSVerse (BD, USA) using anti-human CD34-PE mAb (Miltenyi Biotec, German), follow the identical protocol as described above.
Statistical analysis
One-way analysis of variance (ANOVA) was used for comparisons among the various groups in SPSS program. Results are considered statistically significant when P value is less than 0.05.
Results
Production and specificity of anti-rhIL-3 mAbs
A hybridoma cell culture with high OD reading was selected to be positive for anti-rhIL-3 in ELISA. Several mAb cell lines were selected from this culture using the limiting-dilution method. Culture supernatants from mAb cell lines (C1, and E1) were further analyzed for antibody specificity on Western blots. As shown in Figure 1, the anti-rhIL-3 mAbs did not detect any bands in total E. coli cell lysate (in the absence of IPTG); it also did not cross-react with rhG-CSF, included as a negative control. The antibody did detect a band with expected molecular weight for rhIL-3 (~15 kD) in rhIL-3-expressing E. coli cell lysate (induced with IPTG) and inclusion bodies, as well as the purified rhIL-3. Thus those mAbs are specific antibodies against rhIL-3. The mAb C1, and E1 were subtyped as IgG1 by ELISA analysis (data not shown).
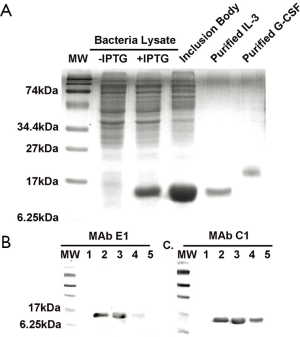
Effects of anti-IL-3 mAbs on ex vivo expansion and differentiation of cord blood CD34+ cells
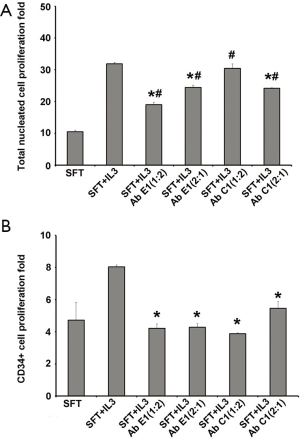
As shown in Figure 2, addition of our lab produced rhIL-3 to the culture medium caused significant increases in CD34+ cells expansion index, from 4.7±1.1 (negative control) to 8.04±0.12 (P<0.05). Increases in CD34+ cells expansion index were also seen when the commercial available rhIL-3 was used (to 7.98±0.42 fold, data not shown). When the anti-IL-3 mAb C1 or E1 was added together with rhIL-3, the expansion index was reduced in an antibody concentration-dependent manner; at a molar ratio of mAb to rhIL-3 equal to or higher than 2, the activity of rhIL-3 was completely blocked. This result indicates that binding of the mAb E1 and C1 to rhIL-3 leads to inhibition of rhIL-3’s biological activity.
Localization of specific mAbs E1 and C1 for synthesized IL-3 fragments by ELISA
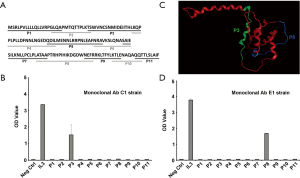
To further define the peptide epitopes of rhIL-3 which were recognized by specific MAbs E1 and C1, a library of 11 peptides covering the full length of rhIL-3 protein were designed and synthesized with 2-5 amino acids overlaps, as indicated in Figure 3A. All the peptides and the rhIL-3 protein were tested by direct ELISA to confirm the binding segment of the specific MAbs. The peptide 3 reacted strongly with MAb C1 (Figure 3B), however, the peptide 8 was recognized by mAb E1 (Figure 3C). A 3-D homology model of rhIL-3 was generated using MOE (Molecular Operation Environment, Chemical Computing Group Inc. Montreal, Canada), indicated in Figure 3D. The locations of peptide 3 and peptide 8 were highlighted, which were found distributed paralleled on the surface of rhIL-3 protein.
Effects of specific synthesized IL-3 fragments on cord blood stem cell ex vivo expansion and differentiation
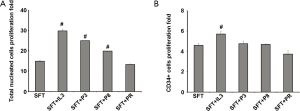
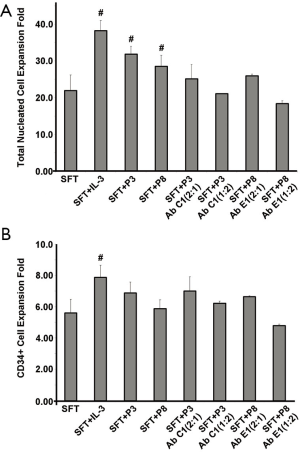
To clarify the bioactivity of the synthesized rhIL-3 peptides, we further carried out the cord blood stem cell expansion experiment, with the addition of 25 ng/mL of the peptide 3, 8 and a random negative peptide. Total MNC number and CD34+ stem cell/progenitors were calculated on day 7. Peptide 3 and peptide 8 increased total nucleated cells expansion index from 14.93±0.55 (SFT control) to 25.01±0.14 and 19.89±0.12, respectively (P<0.05). Whereas the random peptide (PR) did not promote the cell proliferation (13.31±0.33), compared with the SFT basal medium group (Figure 4A). So far, we did not observe any significant difference among the peptides treatment groups and the SFT medium control group, in regarding to CD34+ stem cells/progenitors proliferation fold (Figure 4B). The capability of peptide 3, and 8 on hematopoiesis expansion were neutralized with MAb C1 and MAb E1, respectively, at 0.5 or 2 molar fold to each peptide, in a dose dependent manner (Figure 5).
Discussion
hIL-3 is a four-helix bundle cytokine that is widely expressed in vivo, principally by activated T-lymphocytes, macrophages and stromal cells (14-19). The roles of IL-3 have been extensively investigated. Previously study revealed that, both murine and recombinant gibbon IL-3, support proliferation of multipotential progenitors in culture (20,21). Abundant evidences also suggested that human recombinant IL-3 played a major role in promoting hematopoiesis (22-25), in vitro, as well as hematopoietic derived CD45+ angiogenic cells proliferation (26). In various clinical trials, the therapeutic potential of IL-3 has been demonstrated in patients with chemotherapy and autologous bone marrow transplantation, with accelerated reconstitution of hematopoiesis, and faster regeneration of granulocytes and platelets (27-33). Considering the strong hematopoietic growth-stimulatory activity, rhIL-3 has been adopted to develop a consistent cytokine cocktail for ex vivo expansion of HSCs, therefore, sufficient number of HSCs can be obtained for therapeutic purpose in clinical application, especially with the HSCs isolated from UCB.
Despite the recognition of multipotent stimulatory activity of the rhIL-3, the functional domain of the molecule that participates in promoting hematopoiesis has not yet been identified (19,34). In the present study, we initiated this investigation by using two specific mAbs as well as synthesized peptides for recognizing regions of rhIL-3 involving in hematopoiesis. The two mAbs, specifically against rhIL-3, could abrogate rhIL-3 induced CD34+ cells proliferation and differentiation in a dose dependent manner. This finding suggested that the function domain of rhIL-3 mediating hematopoiesis might be bound or blocked by those mAbs. We further sought the location of function fragment by depicting and synthesizing 11 minimum peptides, which cover the full length of rhIL-3 protein. Among those peptides, we found two that reacted positively to the specific mAbs with ELISA. One is peptide 3, encompasses the 22 amino acids beginning with Thr-28 to Pro-49 of the N-terminal of rhIL-3. The other is peptide 8, encompasses the 20 amino acids spanning from Ala-108 to Arg-127. Moreover, the stimulatory activities of those two peptides were tested in UCB derived CD34+ stem cell culture system, and a favorable result has been achieved, especially with peptide 3. Whereas, peptide 8 presents a partial potency, if any, to promote HSCs proliferation. This finding is of great interesting and consistent with some other investigators observations. Previously, using the approach of targeted saturation mutagenesis, Klein et al. revealed that ten important residues, including Asp21, Gly42, Glu43, Gln45, Asp46, Met49, Arg94, Pro96, Phe113, and Lys116, were mapped to one side of protein and formed a binding site for α subunits of the receptor to mediate the potent bioactivity of IL-3 (19). Among those essential residues, five were located in our short peptide 3, and other three were distributed in the short peptide 8. Considering the data from this lab and the others, we are more likely to believe the functional domains of rhIL-3 are presented as a linear epitope, with bunch of key amino acids residues. However, either of the single peptide presents exactly the same potency to promote hematopoiesis in vitro as the full-length of IL-3, which indicate that the three-dimensional conformation of the protein also functioned. In another word, the function fragment located in peptide 3 and 8 might work synergistically, through a certain type of conformation. Even though, a favorable result has been achieved in the present study to induce UCB derived CD34+ stem cell expansion and differentiation, using the two peptides, compared with the basal medium control. In summary, our findings confirmed the location of the function domain on rhIL-3 to stimulate hematopoiesis, by recognizing two peptides with similar bioactivity, which strongly suggested that the peptides could serve as a replacement for the whole protein in laboratory research, with extremely low cost. Further in vivo investigation will be carried out to evaluate the biological functions of those short peptides on hematopoietic regeneration.
Conclusions
In present study, two mAbs were produced against rhIL-3 with high binding affinity and specificity, which could abrogate rhIL-3 bioactivity on promoting hematopoiesis in vitro. To clarify the function domain of rhIL-3, eleven peptides were depicted and synthesized to cover the whole length of rhIL-3, with 2-5 amino acids overlapped. Among those, peptides 3 and 8 were recognized by the antibodies, using ELISA. CD34+ enriched HSCs, derived from UCB, was employed to detect the biological effects of those peptides. Addition of peptide 3 and 8 to the culture medium induced proliferation and maturation of pluripotent HSCs. Self-renewal of stem cells was not observed in current study. In conclusion, the function domain of rhIL-3 has been identified, and the synthesized peptide containing those function fragment presents a similar bioactivity as rhIL-3 on UCB derived stem cells expansion. It is prospected that the peptides will become an economic replacement for rhIL-3 to be used in laboratory research, and clinical trials.
Acknowledgements
Funding: This work is supported in part by the State New Drug Research & Development (No. 2011ZX09401-027, No. 2011ZX09102-010-04, No. 2013DFA30830, and No. 2014ZX09101042-004).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Chua KN, Chai C, Lee PC, et al. Functional nanofiber scaffolds with different spacers modulate adhesion and expansion of cryopreserved umbilical cord blood hematopoietic stem/progenitor cells. Exp Hematol 2007;35:771-81. [PubMed]
- Czechowicz A, Kraft D, Weissman IL, et al. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science 2007;318:1296-9. [PubMed]
- Szilvassy SJ, Meyerrose TE, Ragland PL, et al. Differential homing and engraftment properties of hematopoietic progenitor cells from murine bone marrow, mobilized peripheral blood, and fetal liver. Blood 2001;98:2108-15. [PubMed]
- Storey JA, Connor RF, Lewis ZT, et al. The transplant iron score as a predictor of stem cell transplant survival. J Hematol Oncol 2009;2:44. [PubMed]
- Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med 1989;321:1174-8. [PubMed]
- Wagner JE, Kurtzberg J. Banking and transplantation of unrelated donor umbilical cord blood: status of the National Heart, Lung, and Blood Institute-sponsored trial. Transfusion 1998;38:807-9. [PubMed]
- Fritsch G, Stimpfl M, Buchinger P, et al. Does cord blood contain enough progenitor cells for transplantation? J Hematother 1994;3:291-8. [PubMed]
- Gluckman E, Rocha V, Arcese W, et al. Factors associated with outcomes of unrelated cord blood transplant: guidelines for donor choice. Exp Hematol 2004;32:397-407. [PubMed]
- Jiang Y, Jiang W, Qiu Y, et al. Effect of a structurally modified human granulocyte colony stimulating factor, G-CSFa, on leukopenia in mice and monkeys. J Hematol Oncol 2011;4:28. [PubMed]
- Xu D. Isolation, characterization, and in vitro propagation of infantile hemangioma stem cells and an in vivo mouse model. J Hematol Oncol 2011;4:54. [PubMed]
- Fan J, Ding X, Jiang Y. A novel monoclonal antibody of human stem cell factor inhibits umbilical cord blood stem cell ex vivo expansion. J Hematol Oncol 2012;5:73. [PubMed]
- Liu XX, Jiang YP. Pharmacokinetic study of a novel recombinant human granulocyte colony-stimulating factor in rats. Chin Med Sci J 2010;25:13-9. [PubMed]
- Jiang Y, Pannell R, Liu JN, et al. Evidence for a novel binding protein to urokinase-type plasminogen activator in platelet membranes. Blood 1996;87:2775-81. [PubMed]
- Arai KI, Lee F, Miyajima A, et al. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem 1990;59:783-836. [PubMed]
- Heimfeld S, Hudak S, Weissman I, et al. The in vitro response of phenotypically defined mouse stem cells and myeloerythroid progenitors to single or multiple growth factors. Proc Natl Acad Sci U S A 1991;88:9902-6. [PubMed]
- Lin Y, Weisdorf DJ, Solovey A, et al. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest 2000;105:71-7. [PubMed]
- Coussens LM, Raymond WW, Bergers G, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev 1999;13:1382-97. [PubMed]
- Itoh K, Platsoucas CD, Balch CM. Autologous tumor-specific cytotoxic T lymphocytes in the infiltrate of human metastatic melanomas. Activation by interleukin 2 and autologous tumor cells, and involvement of the T cell receptor. J Exp Med 1988;168:1419-41. [PubMed]
- Klein BK, Feng Y, McWherter CA, et al. The receptor binding site of human interleukin-3 defined by mutagenesis and molecular modeling. J Biol Chem 1997;272:22630-41. [PubMed]
- Hara T, Miyajima A. Function and signal transduction mediated by the interleukin 3 receptor system in hematopoiesis. Stem Cells 1996;14:605-18. [PubMed]
- Leary AG, Yang YC, Clark SC, et al. Recombinant gibbon interleukin 3 supports formation of human multilineage colonies and blast cell colonies in culture: comparison with recombinant human granulocyte-macrophage colony-stimulating factor. Blood 1987;70:1343-8. [PubMed]
- Sieff CA, Emerson SG, Mufson A, et al. Dependence of highly enriched human bone marrow progenitors on hemopoietic growth factors and their response to recombinant erythropoietin. J Clin Invest 1986;77:74-81. [PubMed]
- Tsai S, Emerson SG, Sieff CA, et al. Isolation of a human stromal cell strain secreting hemopoietic growth factors. J Cell Physiol 1986;127:137-45. [PubMed]
- Winton EF, Srinivasiah J, Kim BK, et al. Effect of recombinant human interleukin-6 (rhIL-6) and rhIL-3 on hematopoietic regeneration as demonstrated in a nonhuman primate chemotherapy model. Blood 1994;84:65-73. [PubMed]
- Bedford Russell AR, Davies EG, Gibson FM, et al. The in vitro effects of granulocyte and granulocyte-macrophage colony-stimulating factor on interleukin-3-dependent proliferation of human neonatal circulating progenitor cells. Pediatr Res 1995;37:630-3. [PubMed]
- Zeoli A, Dentelli P, Rosso A, et al. Interleukin-3 promotes expansion of hemopoietic-derived CD45+ angiogenic cells and their arterial commitment via STAT5 activation. Blood 2008;112:350-61. [PubMed]
- Postmus PE, Gietema JA, Damsma O, et al. Effects of recombinant human interleukin-3 in patients with relapsed small-cell lung cancer treated with chemotherapy: a dose-finding study. J Clin Oncol 1992;10:1131-40. [PubMed]
- Heinzinger M, Waller CF, Rosenstiel A, et al. Quality of IL-3 and G-CSF-mobilized peripheral blood stem cells in patients with early chronic phase CML. Leukemia 1998;12:333-9. [PubMed]
- Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med 1994;331:896-903. [PubMed]
- D'Hondt V, Weynants P, Humblet Y, et al. Dose-dependent interleukin-3 stimulation of thrombopoiesis and neutropoiesis in patients with small-cell lung carcinoma before and following chemotherapy: a placebo-controlled randomized phase Ib study. J Clin Oncol 1993;11:2063-71. [PubMed]
- Lemoli RM, Rosti G, Visani G, et al. Concomitant and sequential administration of recombinant human granulocyte colony-stimulating factor and recombinant human interleukin-3 to accelerate hematopoietic recovery after autologous bone marrow transplantation for malignant lymphoma. J Clin Oncol 1996;14:3018-25. [PubMed]
- Lemoli RM, Fortuna A, Fogli M, et al. Combined use of growth factors to stimulate the proliferation of hematopoietic progenitor cells after autologous bone marrow transplantation for lymphoma patients. Acta Haematol 1996;95:164-70. [PubMed]
- Lemoli RM, Fortuna A, Fogli M, et al. Proliferative response of human marrow myeloid progenitor cells to in vivo treatment with granulocyte colony-stimulating factor alone and in combination with interleukin-3 after autologous bone marrow transplantation. Exp Hematol 1995;23:1520-6. [PubMed]
- Feng Y, Klein BK, McWherter CA. Three-dimensional solution structure and backbone dynamics of a variant of human interleukin-3. J Mol Biol 1996;259:524-41. [PubMed]
Cite this article as: Ren Z, Zhang Y, Zhang Y, Jiang W, Dai W, Ding X, Jiang Y. Influence of IL-3 functional fragment on cord blood stem cell ex vivo expansion and differentiation. Stem Cell Investig 2016;3:6.


