Let-7 regulates cardiomyocyte regeneration
In May of 2015, Kuppusamy et al. described a microRNA (miRNA)-driving maturation process in stem cell-derived cardiomyocytes (CMs) (1). This process elucidated the metabolism shifting through down-regulation of the phosphoinositide 3 kinase (PI3K)/AKT protein kinase/insulin pathway to an up-regulation of fatty acid metabolism, and provided a foundation for potential heart regeneration therapy using human embryonic stem cell-derived cardiomyocytes (hESC-CMs).
The adult human heart is difficult to regenerate after injury. hESCs have the potential to generate innumerable CMs. However, hESC-CMs usually remain in the fetal stage and are not fully functional, which limits their utility for adult-related heart disease studies and constrains their application for clinical therapies. In this report, Dr. Baker’s group identified the Let-7 family of microRNAs, which promoted maturation of hESC-CMs, demonstrating that hESC-CMs have significant value for future basic and applied research in heart regeneration medicine (Figure 1).
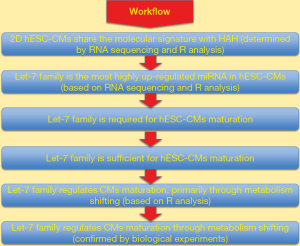
The in vitro maturation process of hESC-CMs described in this paper was well-controlled and convincing. The standard 2D-CMs with 13.5-month culturing (termed 1y-CM) was demonstrated to have a molecular signature similar to that of adult human heart samples, as analyzed by RNA sequencing. R analysis was utilized to classify cell types and analyze cellular pathways. Genome-wide sequencing indicated that the Let-7 family of miRNAs was the most highly expressed miRNA in mature hESC-CMs. This finding was followed by sequential experiments demonstrating that Let-7 is required and sufficient for, and can accelerate, CMs maturation. Beginning with a bioinformatics analysis (R analysis), the authors provided experimental evidence that Let-7 down-regulates the PI3K/AKT protein kinase/insulin pathway and up-regulates fatty acid metabolism, illustrating the basic mechanism of how Let-7 drives the maturation of CMs. Since the Let-7 family of miRNAs contains multiple isoforms, future studies may determine the contribution of other members of the Let-7 family to hESC-CMs maturation and their potential application for heart tissue regeneration.
This report may have broad implications with regard to stemness regulation by the Let-7 family of miRNAs. For example, cancer stem cells have numerous biological traits similar to embryonic stem cells (2), and the PI3K pathway is frequently activated in human cancer (3). Since the Let-7 family has been shown to be highly enriched in cancer exosomes (4), a group of extracellular vesicles that play an important role in intercellular communication (5), the potential metabolism shifting of cancer cells and cancer stem cells by Let-7 is an interesting topic to explore.
The maturation of CMs is complex and related to multiple cellular pathways, which can be challenging to investigate. In this context, this study not only revealed a new mechanism responsible for CMs maturation, but also provided a promising strategy to generate mature CMs. Its deep analysis of gene sequencing data and successful establishment of an in vitro hESC-CMs maturation model also provided a significant contribution to this research area.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kuppusamy KT, Jones DC, Sperber H, et al. Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc Natl Acad Sci U S A 2015;112:E2785-94. [PubMed]
- Zhang M, Li Z, Zhang X, et al. Cancer stem cells as a potential therapeutic target in breast cancer. Stem Cell Investigation 2014;1:14.
- Arcaro A, Guerreiro AS. The phosphoinositide 3-kinase pathway in human cancer: genetic alterations and therapeutic implications. Curr Genomics 2007;8:271-306. [PubMed]
- Ohshima K, Inoue K, Fujiwara A, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One 2010;5:e13247. [PubMed]
- Hannafon BN, Ding WQ. Intercellular communication by exosome-derived microRNAs in cancer. Int J Mol Sci 2013;14:14240-69. [PubMed]
Cite this article as: Xu YF, Ding WQ. Let-7 regulates cardiomyocyte regeneration. Stem Cell Investig 2015;2:23.


