Study of peripheral stem cells mobilization as a treatment line of pediatric dilated cardiomyopathy
Introduction
Dilated cardiomyopathy (DCM) is a myocardial disorder characterized by enlargement and dilation of the left ventricular chamber together with systolic dysfunction that often manifests as congestive heart failure (1). DCM remains the most common form of pediatric cardiomyopathy. Cardiomyopathy is a serious problem in pediatric cardiology despite the relatively low incidence of 0.57 to 2.6 per 100,000 children. One third of patients die within the first year after diagnosis (2,3). In spite of a variety of etiological factors most patients do not possess a demonstrable cause (4).
The conventional medical intervention, including inotropes, dilators and diuretics, is the cornerstone of management; they often provide a short term relief of symptoms but do not improve the outcome of the disease. However, recent clinical studies (3) have suggested bone marrow-derived autologous mononuclear cells or circulating progenitor cells as a promising therapy option for these patients (4,5).
Forty percent of children with symptomatic DCM are resistant to medical treatment (6). Alternative therapeutic options have to be considered for DCM in children with advanced heart failure (7).
Mobilization of bone marrow cells into peripheral blood by certain cytokines such as granulocyte-colony stimulating factor (G-CSF) offers a noninvasive therapeutic strategy for regeneration of myocardium after myocardial infarction (8). However, there are few reports of experimental and clinical studies about the cell mobilization in idiopathic dilated cardiomyopathy (IDCM) in children (7).
Pharmacological mobilization of bone marrow stem cells with G-CSF is well known from clinical hematology. The majority of stem cells mobilized are CD34+, also mesenchymal stem cells (CD34+, CD45+) are mobilized. G-CSF has been used in several clinical trials, because of its noninvasive nature and prolonged stem cell response can be obtained (9).
Bone marrow stem cells were found to contribute to the regeneration of non-hematopoietic organs. Data from preclinical models indicate that cluster of differentiation CD34+ restores the microcirculation and improve myocardial tissue perfusion (4). Recent studies have shown that the granulocyte colony stimulation factor may enhance bone marrow cell migration to damaged heart in increased apoptosis and Fas protein expression (3). G-CSF mobilizes stem cells or progenitor cells from bone marrow go into injured myocardium and accelerates endothelial regeneration, G-CSF also protects cardiomyocytes and endothelial cells from apoptotic cell death. Also G-CSF has been reported to prevent left ventricular remodeling and dysfunction after myocardial infarction (10,11).
One study reported a case of non-ischemic DCM in a patient with thromboangitis obliterans in whom cardiac function improved after G-CSF mobilized peripheral blood mononuclear cell implantation on his ischemic leg. This report suggested that peripheral blood mononuclear implantation with G-CSF could be an effective approach to treating non ischemic heart failure, though the exact mechanisms of improved cardiac function are still unclear (12).
Patients and methods
After ethical committee approval of Faculty of Medicine, Tanta University (code number 1552/12/12, date: 9/1/2013), a randomized clinical trial study was conducted on 40 children with IDCM undergo follow up at Cardiology Unit, Pediatric Department, Tanta University in the period from June 2013 to March 2015. They were divided into two groups: group 1 (20 patients) who received G-CSF together with the traditional lines of treatment and group 2 (20 patients as a control group) received only traditional lines of treatment. An informed consent was taken from parents or guardian of the children among studied groups. Every patient had a special file and code number. Unexpected risks appeared during the course of research cleared to the participants and the Ethical Committee on time with adequate provisions to maintain privacy of all participants and confidentiality of the data.
Inclusion criteria: patients diagnosed with IDCM with ejection fraction less than 45 percent and left ventricular dilatation, kept on anti-failure medications with no improvement of echocardiographic parameters. Exclusion criteria: secondary cardiomyopathy, and cases with cardiomyopathy other than IDCM.
Before treatment all cases were subjected to full history, thorough clinical examination, grading the patients, according to the Modified Ross heart failure (MRHC) classification. Twelve lead electrocardiography to diagnose any associated arrhythmias. Conventional Echo Doppler evaluating the ejection fraction and fractional shortening. Tissue Doppler imaging for assessment of systolic velocity at the mitral annulus (Sm) by GE VIVID 7 apparatus.
Six milliliters of venous blood were collected from each patient by a sterile vein puncture and the sample was contributed in two tubes as follows: two milliliter in the first tube containing EDTA as an anticoagulant for complete blood count (CBC) by ERMA PCE 210N cell counter and for flow cytometry. Assessment of CD34+ cells in peripheral blood was done using flow cytometry technology. The 10 µL phycoerythrin (PE)-conjugated anti-CD34 monoclonal antibody (BD, Kemet medical, Egypt) was added to 100 µL blood samples. As a negative control, a PE-conjugated mouse isotype matched IgG1 antibody (BD, Kemet medical, Egypt) was used. The samples were incubated for 30 min at 4 °C, and then 2 mL of erythrocyte-lysing solution (BD FACslyse) were added. They were kept at room temperature for 10 min and washed twice with PBS. The cell pellet was resuspended in 300 µL 0.5% PFD (paraformaldehyde) solution and kept at 4 °C until use. After staining, the cells were analyzed by a flow cytometer, FACSCalibur (BD, USA) that operates on the basic principle of flow cytometry and analyzes cells according to their size, density and surface marker. One hundred thousand cells were acquired by the flow cytometer. Data collection and analysis were done using CellQuest Pro software (BD, USA). After appropriate gating, the percentage of CD34 positive cells was determined.
Four milliliters were placed in plain tube for estimation of serum lactate dehydrogenase (LDH), total creatinine phosphokinase (CPK), creatinine phosphokinase isoenzyme B (CK-MB) isoenzyme, levels. These parameters were assessed before and after treatment. The sample tubes were labeled with the patient’s name and his serial number.
Group 1 patients were given G-CSF [(Geneleukin) Shangdong Geneleuk Biopharmaceutical Co. Sole agent in Egypt & Middle East United Pharma Company (UPC)] in a dose of 10 mg/kg/day via the subcutaneous route for 5 consecutive days. No concomitant changes were done to anti-failure medication types or doses throughout the study.
After G-CSF treatment: patients of group 1 and group 2 were assessed clinically to obtain MRHC classification and echocardiography at one, three and 6 months from starting the first G-CSF dose in group 1. Assessment of CD34+ cells in peripheral blood was done on both groups at days 0 and 7.
Statistical analysis
Data was collected, coded, and analysis was performed using SPSS software version 21 (Chicago, USA) for Windows. Continuous variables were analyzed as mean values plus or minus standard deviation. Rates and proportions were calculated for categorical data. Paired test was used to compare results before and after treatment (paired data). Bivariate correlation test is to test the association between variables. The level P≤0.05 was considered the cutoff value for significance and P>0.001 was considered as highly significant.
Results
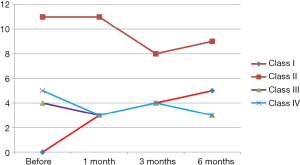
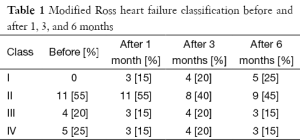
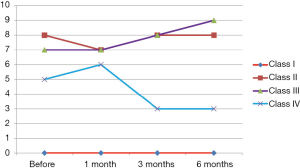
This study was conducted on 20 patients with IDCM (group 1). They were 9 males and 11 females. The mean of their ages in months was 41.74±16.42 months. Twenty children with IDCM with matching age, sex, duration of illness served as a control group (group 2). Results of clinical assessment revealed: (I) improvement of clinical manifestations of two patients among class II who, represent 18.1% of patients among this class and regression of their class to be class I; (II) improvement of one patients among class III who, represent 25% of patients among this class and regression of their class to be class I; (III) improvement of two patients among class IV who represent 40% of patients among this class and regression of their class to be class I. The number of improved patients increased with the duration of follow up at 6 months. So 55% of patients of group 1 (11 patients) and 45% of group 2 (9 patient) were in MRHC classification class II, 20% of group 1 patients (4 patients) and 30% of group 2 patients (6 patients) were in MRHC class III while 25% of group 1 patients (5 patients) and 25% of group 2 patients (5 patients) were in MRHC class IV. There was significant improvement of the MRHC class of group 1 patients (Table 1 and Figure 1). By analysis of results of group 2 patients who received the traditional therapy there was no significant change in MRHC (Figure 2).
Full table
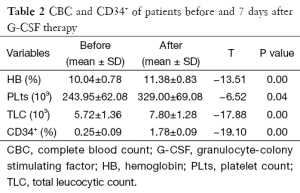
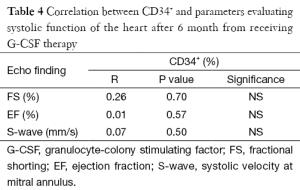
Hemoglobin, platelets and total leucocytic counts (TLC) showed significant elevation after G-CSF therapy (Table 2). Significant improvement was found in echocardiographic data evaluating systolic function of the heart [Ejection fraction, Fractional shortening and systolic velocity at mitral annulus (Sm)] (Table 3). But there was no significant correlation between increased percentage of CD34+ cells and improvement in echocardiographic parameters (Table 4).
Full table

Full table
Full table
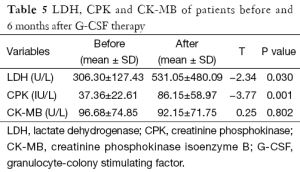
Regarding the results of CPK and LDH, they showed significant increase after treatment while, CK-MB isoenzyme showed non-significant increase in its level after treatment (Table 5).
Full table
Regarding the results of group 2 patients before and after treatment it showed that there is no significant improvement in echocardiographic data even there was a decrease of Sm. For CBC, TLC is the only parameter which showed improvement. Also the results of CK-MB isoenzyme, CPK and LDH, showed significant increase after treatment (Table 6).

Full table
Also as a comparison between of both groups results after treatment all parameters showed significant difference except for LDH and FS (Table 7).

Full table
Discussion
Mobilizing hematopoietic progenitor cells (HPCs) to repair the failing heart in IDCM is a promising intervention to halt the progression of this deadly disease (13).
In the current study, upon treating our patients (group 1) with G-CSF they showed clinical improvement in the form of regression of their MRHC classification. These results were in agreement with Nevin et al., who reported marked clinical improvement in children with DCM in the form of regression of their NYHA classification 7 days from the onset of G-CSF treatment, but in low doses (5 µg/kg/day single S.C dose once daily for 5 consecutive days) (14).
This improvement was also objectively documented by conventional echocardiography and tissue Doppler, which showed improvement of systolic function of the heart in the form of an increased ejection fraction, fractional shortening, and systolic velocity at the mitral annulus (Sm).
Clinical and echocardiographic improvement was not shown in ICDM patients who did not receive G-CSF (group 2). Hüttmann and coworkers showed that G-CSF administration improve physical performance not only in patients with ischemic cardiomyopathy but also in those with DCM (15).
The current study reported a highly significant elevation in white blood cell count, significant elevation in red blood cells, platelet number and CD34+ cells when G-CSF administrated in a dose (10 µg/kg/day single S.C dose once daily for 5 consecutive days). These results were inconsistent with Joseph et al., who reported that G-CSF administration in patients with advanced systolic heart failure was sufficient to produce a significant elevation in white blood cells and to mobilize adequate number of HPCs but in a low dose (5 µg/kg/day single S.C. dose once daily for 5 consecutive days) (13).
The current study showed no significant correlation between the CD34+ cells and the echocardiographic parameters (ejection fraction and fractional shortening) of patients. This result suggests that there is another mechanism by which the G-CSF improves myocardial function in children with IDCM beside the effect of the mobilized bone marrow cells. Hüttmann and coworkers suggested a direct action on the cardiac adrenergic nervous system which may be involved in the effect of G-CSF (15).
In agreement with our study Nevin et al., reported a significant rise in CD34+ cells in the 6th day from the starting dose of G-CSF therapy due to mobilization from the bone marrow into the peripheral blood, and also reported no significant correlation between the percentage of the rise of CD34+ cells and improvement of systolic function of the heart in patients with IDCM after G-CSF administration (14). The underlying mechanism of how mobilized CD34+ contribute to improvement of cardiac remodeling in pediatric DCM patients remains to be elucidated. The best scenario of mobilized cell fate is that cells differentiate into functioning cardiomyocytes within a failing heart, replacing damaged cardiomyocytes. However, studies had shown that the rate of cardiomyocyte differentiation from stem cells is low and the mobilized cells are involved in angiogenesis and host cardiomyocyte regeneration via direct cell differentiation and/or paracrine effects that secrete various growth factors and/or cytokines (12).
In this study there was an elevation of cardiac enzymes, total CK and LDH after G-CSF therapy in comparison to their serum levels before starting treatment. Meneky and coworkers reported that G-CSF treatment promotes temporary elevation of LDH level in rabbit models which returned to normal after discontinuation of G-CSF treatment and suggested that elevation of the LDH during the administration of G-CSF may be related to the differentiation of myeloid progenitors (16).
Mitchell and coworkers reported that there was established relationship between CK, CK-MB and LDH at rest persisted following exercise (17).
So in this study, we suggest the relation between LDH, CK and CK-MB may be due to LDH which catalyzes the conversion of pyruvate to lactate anaerobically to generate adenosine triphosphate (ATP). LDH is present in high concentration in cardiac and skeletal muscle, liver, kidney, lung parenchyma and erythrocytes (9). Also CK catalyzes the conversion of phosphocreatine to creatine releasing high energy phosphate to skeletal and cardiac muscle, creatine is unstable molecule and is converted very rapidly to creatinine, clinical conditions causing elevated serum CK primarily involve skeletal muscle or cardiac tissue, the brain fraction never observed in serum, even after cerebrovascular accident, since the enzyme does not readily cross the blood brain barrier.
These results were in agreement with Joseph et al., who reported elevation of serum creatinine level in one patient after receiving G-CSF (10 µg/kg/day) (13). The CK elevation in our studied cases may be due to G-CSF injection lead to temporary elevation of creatine kinase leading to release of creatine which is an unstable molecule and converted very rapidly to creatinine lead to its elevation which returned to normal level after discontinuation of G-CSF treatment.
Conclusions
Administration of G-CSF may be beneficial in improving systolic functions of the heart in pediatric IDCM. Recommendations: future studies are needed to evaluate precisely the efficacy of this therapy in a large number of patients. Further follow-up by examinations of the patients and echo Doppler are needed to define whether the beneficial effects will be sustained long-term or not.
Acknowledgements
Funding: This work was supported by Tanta University postgraduate and research section, competitive project funding (fund number TU-O7-13-03).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rupp S, Bauer J, Tonn T, et al. Intracoronary administration of autologous bone marrow-derived progenitor cells in a critically ill two-yr-old child with dilated cardiomyopathy. Pediatr Transplant 2009;13:620-3. [PubMed]
- Assmus B, Fischer-Rasokat U, Honold J, et al. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry. Circ Res 2007;100:1234-41. [PubMed]
- Alvarez J, Wilkinson JD, Lipshultz SE. The Pediatric Cardiomyopathy Registry Study Group. Outcome predictors for pediatric dilated cardiomyopathy: A systemic review. Prog Pediatr Cardiol 2007:25-32. [PubMed]
- Selem SM, Kaushal S, Hare JM. Stem cell therapy for pediatric dilated cardiomyopathy. Curr Cardiol Rep 2013;15:369. [PubMed]
- Olguntürk R, Kula S, Sucak GT, et al. Peripheric stem cell transplantation in children with dilated cardiomyopathy: preliminary report of first two cases. Pediatr Transplant 2010;14:257-60. [PubMed]
- Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med 2007;167:989-97. [PubMed]
- Dufour DR, Lott JA, Henry JB. Clinical enzymology. In: Henry JB, editor. clinical diagnosis and management by laboratory methods. 20thed. Philadelphia: WB Sounders; 2001:281-303.
- Tsirka AE, Trinkaus K, Chen SC, et al. Improved outcomes of pediatric dilated cardiomyopathy with utilization of heart transplantation. J Am Coll Cardiol 2004;44:391-7. [PubMed]
- Zohlnhöfer D, Ott I, Mehilli J, et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients with acute myocardial infarction: a randomized controlled trial. JAMA 2006;295:1003-10. [PubMed]
- Zohlnhöfer D, Dibra A, Koppara T, et al. Stem cell mobilization by granulocyte colony-stimulating factor for myocardial recovery after acute myocardial infarction: a meta-analysis. J Am Coll Cardiol 2008;51:1429-37. [PubMed]
- Harada M, Qin Y, Takano H, et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med 2005;11:305-11. [PubMed]
- Kakihana A, Ishida A, Miyagi M, et al. Improvement of cardiac function after granulocyte-colony stimulating factor-mobilized peripheral blood mononuclear cell implantation in a patient with non-ischemic dilated cardiomyopathy associated with thromboangiitis obliterans. Intern Med 2009;48:1003-7. [PubMed]
- Joseph J, Mehta P, Rimawi A, et al. Stem cell mobilization utilizing granulocyte colony stimulating factor in advanced chronic heart failure. Lessons from a pilot study. Eur Heart J 2008;10:K24-6.
- Habeeb NM, Youssef OI, El Hadidi ES. Therapeutic role of mobilized bone marrow cells in children with nonischemic dilated cardiomyopathy. ISRN Pediatr 2012;2012:927968.
- Hüttmann A, Dührsen U, Stypmann J, et al. Granulocyte colony-stimulating factor-induced blood stem cell mobilisation in patients with chronic heart failure--Feasibility, safety and effects on exercise tolerance and cardiac function. Basic Res Cardiol 2006;101:78-86. [PubMed]
- Menekay S, Ozsan GH, Demirkan F, et al. Effect of granulocyte-colony-stimulating factor on serum lactate dehydrogenase levels and isoenzymes in a rabbit model. Acta Haematol 2002;107:18-22. [PubMed]
- Kanter MM, Lesmes GR, Kaminsky LA, et al. Serum creatine kinase and lactate dehydrogenase changes following an eighty kilometer race. Relationship to lipid peroxidation. Eur J Appl Physiol Occup Physiol 1988;57:60-3. [PubMed]
Cite this article as: El-Shanshory M, El-Shehaby W, Hables N, Hamad S, Attia M, El-Said A. Study of peripheral stem cells mobilization as a treatment line of pediatric dilated cardiomyopathy. Stem Cell Investig 2015;2:21.