
The effect of short-term necrostatin-1 treatment on the differentiation of human induced pluripotent stem beta cells
Introduction
Type 1 diabetes mellitus (T1DM) is T-cell moderated disorder characterized by autoimmune destruction of insulin-producing pancreatic beta cells, resulting in severe insulin deficiency, hyperglycemia, and metabolic disorientation (1). Patients suffering from hyperglycemia as a result of T1DM experience manifestations of discomforting symptoms including polydipsia, polyuria, and diabetic ketoacidosis that could lead to death (1). To ameliorate the threatening consequences of insulin deficiency, daily self-administered exogenous insulin therapy is being used to rectify the lack of insulin produced (2). Although this method can regulate blood glucose levels, variability in the suboptimal absorption of subcutaneously absorbed insulin and imprecise dosing to achieve proper glycemic control have been reckoned as an impediment to this method of treatment (3).
To relieve patients of life-long exogenous insulin therapy, pancreatic islet transplantation has been explored as a promising cure for T1DM (4). Islet allotransplantation has proven to be a propitious method of beta cell replacement that can induce glycemic balance and healthy insulin production (5). Established in 2000 as the Edmonton Protocol, allotransplantation of pancreatic islet cells is performed through preparation of pancreases from deceased donors and transplantation into recipients to achieve sufficient glycemic control for extended periods of time (6). In 2000, seven patients who received islet allografts in conjunction with a glucocorticoid-free immunosuppressive regimen were able to sustain insulin dependency and euglycemia, an unprecedented success rate in the world of clinical islet transplantation (6). In 2007, islet cell allotransplantation was proven to successfully restore long-term endogenous insulin production and glycemic control in T1DM patients. Despite the achievements made, insulin independence was found to be gradually lost over time (7). Furthermore, islet transplantation requires islets from 2–4 donors per recipient in order to promote the engraftment of sufficient insulin-producing cells to achieve insulin independence. Due to the critical scarcity of donors and imperfect isolation efforts, few patients with T1DM have been able to obtain islet allografts to restore euglycemia (8).
Efforts to address the shortage of donors have introduced studies involving the utilization of human induced pluripotent stem cells (hiPSC) to produce mature insulin-producing beta cells. HiPSCs are cells derived from somatic cells through a method of reprogramming to induce conversion into pluripotent embryonic stem cells (9). HiPSCs provide the advantage of eliminating immune rejection through the utilization of patient-specific cells (10). This has, however, been an ongoing progress. Functional beta cells derived from hiPSCs have been evidenced to display an increase in the glucose threshold for insulin secretion (11). The protocol to generate mature insulin-producing beta cells from hiPSCs to successfully demonstrate such function of endogenous beta cell in vitro has yet to be found. Insulin expressing cells derived from hiPSCs express insulin lack the glucose responsiveness characteristic of mature endogenous beta cells (11,12). Failure to devise a successful hiPSC differentiation protocol to give rise to mature insulin-producing beta cells has resulted in the conduction of a myriad of studies to find the missing piece to current targeted differentiation protocols (11,13,14). Pathway analysis has revealed that the LXR/RXR pathway was inhibited in S7 hiPS beta cells, associating with a high proliferation index as indicated by an increased Ki67 staining compared to human islet cells (15). The Wnt/beta-catenin and PCP signaling pathways have also been identified as promising targets for improving in vitro beta cell maturation (15,16). Despite strong evidence suggesting that Wnt signaling could induce the final maturation of S7 hiPS beta cells, a recent study has shown that Wnt modulation at the S7 stage of hiPSCs only modestly promoted beta-cell-like maturation with no improvement in the glucose-stimulated insulin secretion (17). Thus, efforts must be made to identify molecules that could promote the final differentiation step of hiPSCs into mature functional beta cells.
Necrostatin-1 (Nec-1) is a small-molecule allosteric inhibitor of the death domain receptor-interacting protein kinase 1 (RIPK1) (18). The protective effects of short-term Nec-1 treatment on preventing necroptosis and reducing cell death in multiple cell types and animal models of ischemic injury have been studied extensively (19,20). However, the function of Nec-1 in pancreatic islets and beta cells has not been well characterized. In one study, Nec-1 treatment of beta cells lines and isolated mouse islets for 24 hours decreased the release of DAMPs and improved cell survival after nitric oxide exposure (21). Our recent study has demonstrated the novel effects of 3-day Nec-1 treatment to enhance the islet insulin content, pancreatic endocrine cell differentiation, GLUT2 expression, and insulin secretion of young porcine islets (22). Furthermore, prolonged 7-day culture with Nec-1 did not significantly improve the level of beta cells and insulin secretion stimulation index (SI) compared to 3-day culture (22). As young porcine islets have been reported to contain a major stem-cell like population similar to hiPSCs that is capable of differentiating into mature endocrine cells, we hypothesized that short-term exposure to Nec-1 may affect the in vitro differentiation of hiPSCs into mature functional beta cells (23). In this present study, we aimed to determine whether short-term Nec-1 treatment during in vitro culture could improve the differentiation and function of hiPS beta cells.
Materials and methods
Cell source, culture, and Nec-1 treatment
HiPS beta cells, derived from ChiPSC22 cells that were reprogrammed from normal healthy skin fibroblasts, was purchased from Cellartis (cat# Y10100, Takara Bio, Mountain View, CA, USA). Cryopreserved hiPS beta cells were thawed and cultured for maturation according to the manufacturer’s protocol. In brief, thawed cells were cultured in Beta Cell Maintenance Medium at a cell density of 2.0×105 viable cells/cm2 in a 37 °C, 5% CO2 humidified incubator (cat# 3110, Thermo Forma Series II 3120 Water Jacketed CO2 Incubators, Carlsbad, CA, USA). On day 12 of culture, cells were divided into 2 groups for culture in Beta Cell Medium 2 alone (n=3) or supplemented with Nec-1 (100 µM, Abcam, Cambridge, UK, cat# ab141053, n=3) until day 15 (22). A full media change was performed on day 1 after thawing and every other day thereafter. Cells were collected after thawing, before Nec-1 treatment (day 12 of culture) and after Nec-1 treatment (day 15 of culture) for assessment.
Flow cytometry
Flow cytometry was used to characterize hiPS beta cells after thawing, before Nec-1 treatment, and after Nec-1 treatment as previously described (22). HiPS beta cells were stained with 7-AAD viability dye (7-aminoactinomycin D; cat# A1310, Invitrogen, Carlsbad, CA, USA) for 30 minutes on ice after thawing, before Nec-1 treatment, and after Nec-1 treatment. Stained cells were fixed in 4% paraformaldehyde for 10 minutes and permeabilized using Intracellular Staining Permeabilization Wash Buffer (cat# 421002, BioLegend, San Diego, CA, USA) for 15 minutes on ice. After permeabilization, cells were incubated in Protein Block (cat# ab64226, Abcam) for 30 minutes on ice to reduce non-specific binding. Blocked cells were stained with fluorescently conjugated antibodies for 30 minutes on ice in Intracellular Staining Permeabilization Wash Buffer (cat# 421002, BioLegend) supplemented with 0.5% bovine serum albumin (cat# BAL62-0500, Equitech-Bio, Inc., Kerrville, TX, USA). PE conjugated anti-insulin (anti-insulin-PE; cat# 8508, CST, Danvers, MA, USA) antibody was used as a marker for beta-cells. APC conjugated anti-glucagon (anti-glucagon-APC; cat# NBP2-21803AF647, Novus Biological, Littleton, CO, USA) antibody was used as a marker for alpha-cells. FITC-conjugated anti-GLUT2 (cat# FAB1414G-100UG, Novus Biological) and PE conjugated anti-insulin (cat# 8508, CST) antibodies were used to identify GLUT2-positive beta cells. After staining, cells were analyzed on a NovoCyte 3000VYB Flow Cytometer (ACEA Biosciences, Inc., San Diego, CA, USA) and quantified using FlowJo software (FlowJo, Ashland, OR, USA). Gating controls included unstained, single-stained, fluorescence minus one, and matching isotype samples.
Glucose-stimulated insulin secretion
The insulin secretion in response to glucose stimulation of hiPS beta cells after thawing, before Nec-1 treatment, and after Nec-1 treatment was determined using glucose-stimulated insulin release assay (22). HiPS beta cells were incubated at 37 °C and 5% CO2 for 1 hour in the following order: low glucose #1 (2.8 mM; L1), high glucose (28 mM; H), low glucose #2 (2.8 mM; L2), and high glucose plus 3-isobutyl-1-methylxanthine (28 mM + 0.1 mm IBMX; H+) media. The insulin secreted by hiPS beta cells in each glucose media was analyzed using a standard human insulin enzyme-linked immunosorbent assay (Human Insulin ELISA; cat# 10-1113-01, Mercodia, Winston Salem, NC, USA) and quantified on a microplate reader (Infinite F200, Tecan and Magellan V7, Männedorf, Switzerland). The amount of insulin was normalized to the DNA content of each sample and expressed as pg of insulin/ng of DNA/h. The SI was calculated as the ratio of the amount of insulin secreted in the high glucose media (H) over the amount of insulin secreted in the first low glucose media (L1).
DNA content
HiPS beta cells were lysed, and the DNA content was analyzed as previously described (24). In brief, hiPS beta cells were incubated in cell lysis buffer (10 mM Tris-HCl, 1 mM EDTA, 1% Triton X-100, pH 8) and sonicated (Sonics VibraCell Ultrasonic Processor Model VC70T; Sonics & Materials, Inc, Newtown, CT, USA) for 30 seconds on ice. After centrifugation, the DNA content in the supernatant was quantified using a fluorescent DNA stain (Quant-iT PicoGreen dsDNA kit; cat# Q32850, Molecular Probes, Eugene, OR, USA) and read on a microplate reader (Infinite F200, Tecan and Magellan V7, Männedorf, Switzerland).
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). A one-way ANOVA followed by a post hoc Tukey’s HSD test was used to determine statistical significance. A P value <0.05 was considered to be statistically significant. Statistical analysis was performed on GraphPad Prism (GraphPad Software 8.0.1, San Diego, CA, USA).
Results
Characterization of hiPS beta cells after thawing
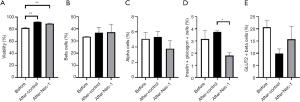
Flow cytometry was used to characterize the viability and cellular content of hiPS beta cells after thawing. The viability of hiPS beta cells after thawing was 87.70%±0.52% (Table 1). The beta-cell and alpha-cell composition was 39.60%±1.16% and 3.62%±0.17%, respectively (Table 1). The level of insulin- and glucagon-positive bi-hormonal cells after thawing was 2.10%±0.32% (Table 1). The percentage of GLUT2-positive beta cells was 13.26%±5.51% (Table 1).

Full table
Characterization of hiPS beta cells before and after short-term Nec-1 treatment
Both untreated (91.73%±1.05%) and Nec-1-treated hiPS beta cells (89.13%±0.85%) on day 15 of culture had a significantly higher viability than hiPS beta cells before Nec-1 treatment (81.83%±1.16%) on day 12 of culture (P<0.01, Figure 1A). There was no significant difference in the beta-cell composition of hiPS beta cells before Nec-1 treatment (33.60%±0.21%) and after short-term Nec-1 treatment (control: 36.83%±4.23%, Nec-1 treated: 37.23%±6.56%) (P=NS, Figure 1B). Similarly, no significant difference in the level of alpha cells was observed in hiPS beta cells before Nec-1 treatment (5.08%±0.82%) and after 3-day culture in either control (5.32%±0.74%) or Nec-1-treated media (3.79%±1.06%) (P=NS, Figure 1C). On day 15 of culture, hiPS beta cells cultured in media supplemented with Nec-1 (1.80%±0.25%) had a significantly lower composition of insulin- and glucagon-positive bi-hormonal cells compared to untreated hiPS beta cells (3.75%±0.083%) (P<0.05, Figure 1D). The percentage of insulin- and glucagon-positive bi-hormonal cells in hiPS beta cells before Nec-1 treatment (3.16%±0.70%) was similar to hiPS beta cells cultured in either control or Nec-1 treated media on day 15 of culture (P=NS, Figure 1D). There was no significant difference in the composition of GLUT2-positive beta cells between hiPS beta cells before (20.67%±2.75%) and after short-term Nec-1 treatment (control: 9.90%±1.86%, Nec-1 treated: 15.73%±5.23%) (P=NS, Figure 1E).
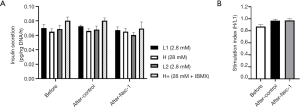
Glucose-stimulated insulin secretion of hiPS beta cells before and after short-term Nec-1 treatment
Nec-1 treated hiPS beta cells (L1: 0.067±0.0044 pg insulin/ng DNA/h, H: 0.065±0.0042 pg insulin/ng DNA/h, L2: 0.060±0.0037 pg insulin/ng DNA/h, H+: 0.070±0.0091 pg insulin/ng DNA/h) secreted a similar amount of insulin during glucose stimulation in comparison to control hiPS beta cells (L1: 0.073±0.0017 pg insulin/ng DNA/h, H: 0.066±0.0031 pg insulin/ng DNA/h, L2: 0.068±0.0049 pg insulin/ng DNA/h, H+: 0.080±0.0037 pg insulin/ng DNA/h) and hiPS beta cells before Nec-1 treatment (L1: 0.070±0.0051 pg insulin/ng DNA/h, H: 0.065±0.0042 pg insulin/ng DNA/h, L2: 0.069±0.0053 pg insulin/ng DNA/h, H+: 0.080±0.0050 pg insulin/ng DNA/h) (P=NS, Figure 2A). The SI was also similar between hiPS beta cells before (0.87±0.036) and after short-term Nec-1 treatment (control: 0.97±0.026, Nec-1 treated: 0.97±0.028) (P=NS, Figure 2B).
Discussion
Inducing the final differentiation of hiPSCs into functionally mature beta cells still remains a challenge even with the current state-of-the-art differentiation protocol. Thus, the identification of a signaling pathway or a molecule to promote the final step of beta-cell differentiation is vital to generate mature insulin-producing beta cells from hiPSCs. We have previously reported that Nec-1 treatment during short-term culture can facilitate pancreatic endocrine cell differentiation and enhance GLUT2 expression, leading to an improvement in insulin secretion of young porcine islets (22). In the present study, we are the first to examine the effect of Nec-1 on the differentiation and function of hiPS beta cells after short-term in vitro culture. We found that short-term Nec-1 treatment reduced the percentage of insulin- and glucagon-positive bi-hormonal cells; however, the viability and composition of beta cells, alpha cells and GLUT2-positive beta cells remained unchanged. Furthermore, Nec-1 supplementation to hiPS beta-cell differentiation media did not affect glucose-stimulated insulin secretion.
Our findings that short-term Nec-1 treatment did not affect the viability of hiPS beta cells supports our previous results that Nec-1 did not alter the viability of young pre-weaned porcine islets after 3-day culture (22,24). This could be due to the high viability in both control and Nec-1 treated groups as the viability was higher than 85% on average. In accordance with our findings, the supplementation of Nec-1 to the culture media of encapsulated human islets in either low- or normal-nutrient culture condition at 20% oxygen level did not significantly improve nuclear DNA content, a measurement of cell survival (25). Another study has similarly demonstrated that the addition of Nec-1 did not affect the percentage of dead cells in normal culture condition after 24 hours as assessed by Sytox staining in βTC-6 and INS-1/832 pancreatic beta cells as well as mouse islet cells (21). However, Nec-1 has been shown to promote islet cell survival from stress induced by low oxygen culture condition or nitric oxide donors (25). Beta cells are exposed to considerable stress that is partially contributed by DAMPs after transplantation, leading to substantial loss of beta cell mass and poor engraftment outcomes (26,27). As Nec-1 has been shown to significantly reduce the release of DAMPs, examining the use of Nec-1 on hiPS beta cells under stressors that mimic the post-transplant environment may help to elucidate the effects of Nec-1 on the viability of hiPS beta cells after transplantation (21,25).
Various studies have attempted to improve the differentiation of hiPSCs into beta cells during in vitro culture (28). A recent study has demonstrated that, even with the current state-of-the-art protocol, differentiating hiPSC-derived S6 cells into S7 cells through incubation in S7 media could not improve the beta-cell content (17). In parallel, our present findings showed that the percentage of beta cells remained unchanged after culturing hiPS beta cells in beta-cell differentiation medium. Similar to previously published work, the percentage of alpha cells in our study was not improved after differentiation culture (17).
In contrary to our previous findings that Nec-1 induced the differentiation of endocrine cells in young pre-weaned porcine islets after 3-day culture, the supplementation of Nec-1 to the beta-cell differentiation medium did not impact the percentage of beta cells and alpha cells after 3 days of culture in the present study (22). A possible reason for these results is that the use of a small-molecule differentiation inducer during the final step of differentiation of hiPSCs may require more than 3 days to be effective. Thatava et al. have utilized DAPT, HGF, IGF-1, and GLP-1 to differentiate hiPSCs into islet-like cells over 6 days of culture (29). In an attempt to generate insulin producing cells, Walczak et al. have cultured hiPSCs in medium supplemented with SB431542, dorsomorphin, and retinoic acid for 7 days (30). During the final differentiation step, Rezania et al. incubated hiPSCs in medium containing ITS-X, T3, ALK5 inhibitor II, trolox, and AXL inhibitor R428 for 7 to 15 days (31). Another plausible explanation for the unchanged level of beta cells is that islets are exposed to considerable damages caused by prolonged ischemic time, pancreas preservation, and islet isolation (32,33). These stresses lead to the release of pro-inflammatory mediators, contributing to not only islet inflammation and islet demise but also the dedifferentiation of beta cells (34,35). Exposure of the pro-inflammatory cytokine IL-1β to both rat and human islets caused beta-cell dedifferentiation as indicated by decreased mRNA expression of insulin and impaired insulin secretion capacity (36). The combinations of pro-inflammatory cytokines, IL-1β + IFN-γ and TNF-α + IFN-γ, could reduce the expression of genes responsible for glucose-stimulated insulin secretion and maturation in purified beta cells from rat islets (37). As short-term Nec-1 exposure has been shown to inhibit the release of DAMPs that promote inflammatory responses, it is possible that Nec-1 treatment assisted in the differentiation of young pre-weaned porcine islets over 3-day culture by blocking the upregulation of inflammatory pathways induced by pancreas procurement and islet isolation (21,22,25). Since hiPSCs are exposed to substantially less insults and inflammation throughout the differentiation process, the ability of Nec-1 to facilitate endocrine cell differentiation might not have been as effective compared to our previous study.
The short-term treatment of Nec-1 in the present study resulted in a significant decrease in the percentage of insulin- and glucagon-positive bi-hormonal cells, but no significant changes in the level of insulin-positive or glucagon-positive cells. A previous study has demonstrated similar results that while differentiating S6 hiPSCs to S7 hiPSCs reduced the bi-hormonal cells, the amount of beta- and alpha cells remained unchanged (17). Furthermore, the treatment of S7 hiPSCs with a tankyrase inhibitor for 48 hours could only lower the level of bi-hormonal cells but not the percentage of insulin-positive or glucagon-positive cells normalized to total DAPI-positive cells (17). In accordance with the unaltered level of beta cells after short-term Nec-1 treatment, the insulin secretion capacity of Nec-1 treated hiPS beta cells was similar to untreated cells. These observations are in line with previous findings that Wnt-modulation and tankyrase inhibition could not improve glucose-stimulated insulin secretion of S7 hiPSCs even though the levels of bi-hormonal cells were altered (17). Our results and previous work done by others highlight the importance of assessing both the differentiation of beta cells and the insulin secretion ability in the search for a small-molecule differentiation inducer to promote the final maturation step of hiPSCs. We believe that elucidating the mechanism by which Nec-1 reduced the level of bi-hormonal cells may be helpful to determine whether prolonging Nec-1 treatment can promote the last differentiation step of hiPSCs into functional beta cells. The effect of Nec-1 has also been demonstrated to be concentration-dependent, and concentrations ranging from 10 to 300 uM have been utilized on different cell types (19,38-40). These studies suggest that different concentrations of Nec-1 should be evaluated on hiPS beta cells.
In conclusion, the current study has, to our knowledge, provided the first analysis of short-term Nec-1 treatment on the differentiation and function of hiPS beta cells during the final in vitro maturation stage. While the content of beta cells and insulin secretion capacity of hiPS beta cells remained unchanged, Nec-1 supplementation during short-term culture significantly reduced the level of insulin- and glucagon-positive bi-hormonal cells. Future studies optimizing the concentration and treatment duration of Nec-1 will be helpful to further determine whether Nec-1 can promote the final differentiation step of hiPSCs into functional mature beta cells.
Acknowledgments
Funding: This manuscript was supported with internal funds from the Department of Surgery, University of California, Irvine.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/sci.2020.04.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014;383:69-82. [Crossref] [PubMed]
- Shapiro AM. Islet transplantation in type 1 diabetes: ongoing challenges, refined procedures, and long-term outcome. Rev Diabet Stud 2012;9:385-406. [Crossref] [PubMed]
- Gradel AKJ, Porsgaard T, Lykkesfeldt J, et al. Factors affecting the absorption of subcutaneously administered insulin: effect on variability. J Diabetes Res 2018 Jul 4;2018:1205121.
- Rickels MR, Robertson RP. Pancreatic islet transplantation in humans: recent progress and future directions. Endocr Rev 2019;40:631-68. [Crossref] [PubMed]
- Bottino R, Knoll MF, Knoll CA, et al. The future of islet transplantation is now. Front Med (Lausanne) 2018;5:202. [Crossref] [PubMed]
- Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230-8. [Crossref] [PubMed]
- Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318-30. [Crossref] [PubMed]
- Rother KI, Harlan DM. Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Invest 2004;114:877-83. [Crossref] [PubMed]
- Georgomanoli M, Papapetrou EP. Modeling blood diseases with human induced pluripotent stem cells. Dis Model Mech 2019. [Crossref] [PubMed]
- Singh VK, Kalsan M, Kumar N, et al. Induced pluripotent stem cells: applications in regenerative medicine, disease modeling, and drug discovery. Front Cell Dev Biol 2015;3:2. [Crossref] [PubMed]
- Blum B, Hrvatin S, Schuetz C, et al. Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat Biotechnol 2012;30:261-4. [Crossref] [PubMed]
- Johnson JD. The quest to make fully functional human pancreatic beta cells from embryonic stem cells: climbing a mountain in the clouds. Diabetologia 2016;59:2047-57. [Crossref] [PubMed]
- Russ HA, Parent AV, Ringler JJ, et al. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J 2015;34:1759-72. [Crossref] [PubMed]
- Petersen MBK, Azad A, Ingvorsen C, et al. Single-cell gene expression analysis of a human ESC model of pancreatic endocrine development reveals different paths to β-cell differentiation. Stem Cell Reports 2017;9:1246-61. [Crossref] [PubMed]
- Vethe H, Bjørlykke Y, Ghila LM, et al. Probing the missing mature β-cell proteomic landscape in differentiating patient iPSC-derived cells. Sci Rep 2017;7:4780. [Crossref] [PubMed]
- Bader E, Migliorini A, Gegg M, et al. Identification of proliferative and mature β-cells in the islets of Langerhans. Nature 2016;535:430-4. [Crossref] [PubMed]
- Vethe H, Ghila L, Berle M, et al. The effect of Wnt pathway modulators on human iPSC-derived pancreatic beta cell maturation. Front Endocrinol (Lausanne) 2019;10:293. [Crossref] [PubMed]
- Degterev A, Hitomi J, Germscheid M, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 2008;4:313-21. [Crossref] [PubMed]
- Degterev A, Huang Z, Boyce M, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 2005;1:112-9. [Crossref] [PubMed]
- Shen B, Mei M, Pu Y, et al. Necrostatin-1 attenuates renal ischemia and reperfusion injury via meditation of HIF-1α/mir-26a/TRPC6/PARP1 signaling. Mol Ther Nucleic Acids 2019;17:701-13. [Crossref] [PubMed]
- Tamura Y, Chiba Y, Tanioka T, et al. NO donor induces Nec-1-inhibitable, but RIP1-independent, necrotic cell death in pancreatic β-cells. FEBS Lett 2011;585:3058-64. [Crossref] [PubMed]
- Lau H, Corrales N, Alexander M, et al. Necrostatin-1 supplementation enhances young porcine islet maturation and in vitro function. Xenotransplantation 2020;27:e12555. [Crossref] [PubMed]
- Li WC, Chen CY, Kao CW, et al. Porcine neonatal pancreatic cell clusters maintain their multipotency in culture and after transplantation. Sci Rep 2018;8:8212. [Crossref] [PubMed]
- Qi M, Bilbao S, Forouhar E, et al. Encompassing ATP, DNA, insulin, and protein content for quantification and assessment of human pancreatic islets. Cell Tissue Bank 2018;19:77-85. [Crossref] [PubMed]
- Paredes-Juarez GA, Sahasrabudhe NM, Tjoelker RS, et al. DAMP production by human islets under low oxygen and nutrients in the presence or absence of an immunoisolating-capsule and necrostatin-1. Sci Rep 2015;5:14623. [Crossref] [PubMed]
- Itoh T, Takita M, SoRelle JA, et al. Correlation of released HMGB1 levels with the degree of islet damage in mice and humans and with the outcomes of islet transplantation in mice. Cell Transplant 2012;21:1371-81. [Crossref] [PubMed]
- Itoh T, Iwahashi S, Kanak MA, et al. Elevation of high-mobility group box 1 after clinical autologous islet transplantation and its inverse correlation with outcomes. Cell Transplant 2014;23:153-65. [Crossref] [PubMed]
- Shahjalal HM, Abdal Dayem A, Lim KM, et al. Generation of pancreatic β cells for treatment of diabetes: advances and challenges. Stem Cell Res Ther 2018;9:355. [Crossref] [PubMed]
- Thatava T, Nelson TJ, Edukulla R, et al. Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny. Gene Ther 2011;18:283-93. [Crossref] [PubMed]
- Walczak MP, Drozd AM, Stoczynska-Fidelus E, et al. Directed differentiation of human iPSC into insulin producing cells is improved by induced expression of PDX1 and NKX6.1 factors in IPC progenitors. J Transl Med 2016;14:341. [Crossref] [PubMed]
- Rezania A, Bruin JE, Arora P, et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol 2014;32:1121-33. [Crossref] [PubMed]
- Contreras JL, Eckstein C, Smyth CA, et al. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes 2003;52:2935-42. [Crossref] [PubMed]
- Miki A, Ricordi C, Yamamoto T, et al. Improved human islet preparations using glucocorticoid and exendin-4. Pancreas 2014;43:1317-22. [Crossref] [PubMed]
- Cardozo AK, Heimberg H, Heremans Y, et al. A comprehensive analysis of cytokine-induced and nuclear factor-kappa B-dependent genes in primary rat pancreatic beta-cells. J Biol Chem 2001;276:48879-86. [Crossref] [PubMed]
- Sun J, Ni Q, Xie J, et al. β-cell dedifferentiation in patients with T2D with adequate glucose control and nondiabetic chronic pancreatitis. J Clin Endocrinol Metab 2019;104:83-94. [PubMed]
- Nordmann TM, Dror E, Schulze F, et al. The role of inflammation in β-cell dedifferentiation. Sci Rep 2017;7:6285. [Crossref] [PubMed]
- Ortis F, Naamane N, Flamez D, et al. Cytokines interleukin-1beta and tumor necrosis factor-alpha regulate different transcriptional and alternative splicing networks in primary beta-cells. Diabetes 2010;59:358-74. [Crossref] [PubMed]
- Takahashi N, Duprez L, Grootjans S, et al. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis 2012;3:e437. [Crossref] [PubMed]
- Zheng HW, Chen J, Sha SH. Receptor-interacting protein kinases modulate noise-induced sensory hair cell death. Cell Death Dis 2014;5:e1262. [Crossref] [PubMed]
- Liang S, Lv ZT, Zhang JM, et al. Necrostatin-1 attenuates trauma-induced mouse osteoarthritis and IL-1β induced apoptosis via HMGB1/TLR4/SDF-1 in primary mouse chondrocytes. Front Pharmacol 2018;9:1378. [Crossref] [PubMed]
Cite this article as: Lau H, Park S, Luong C, Corrales N, Rodriguez S, Li S, Alexander M, Lakey JRT. The effect of short-term necrostatin-1 treatment on the differentiation of human induced pluripotent stem beta cells. Stem Cell Investig 2020;7:6.


