
Cord blood transplantation for bone marrow failure syndromes: state of art
Introduction
Bone marrow failure (BMF) disorders are rare diseases, which can occur in children and adults, as a consequence of idiopathic [aplastic anemia (AA)] or inherited disorders [such as Fanconi anemia (FA), Diamond-Blackfan anemia (DBA), dyskeratosis congenita (DC), and others]. Hematopoietic stem cell transplantation (HSCT) from a human leukocyte antigen (HLA)-matched sibling donor (MSD) using bone marrow (BM) as stem cell source, represents the first-line treatment option for all patients aged less than 40 with either inherited or acquired BMF (1-4). Appropriate classification and diagnosis of patients is mandatory, because of its impact on clinical management, choice of stem cell source and preparative regimen in case of HSCT, estimated risk for complications, including future cancers, genetic and medical counselling as well as follow-up of patients and family members (5).
Immunosuppressive therapy (IST) is the treatment of choice in case of lack of a sibling donor and for patients diagnosed with idiopathic BMF over 40 years of age (2,6). Growth factors, corticosteroids, and androgens represent the main alternative, non-transplant therapies for inherited BMF but results are often heterogeneous (5,7-12).
In historical cohorts, the use of alternative donors increased the risk of poor outcomes (4,13,14). However, more recently, with advances in HLA typing and a better choice of conditioning regimens as well as the improvement in supportive care, long-term survival after an HSCT from unrelated donors, has significantly improved (15,16). Therefore, in case of a lack of a suitable BM donor or failure of first-line IST, other alternative stem cell sources and donors can be considered. In this setting, cord blood (CB) offers an alternative, rapidly available, source of hematopoietic stem cells (HSC).
In this special issue, we have reviewed outcomes and results of principal studies concerning cord blood transplantation (CBT) in the setting of both idiopathic and inherited BMF disorders, addressing particular attention to FA.
First proof of concept
The first data on the infusion of CB cells in humans (17) arose from a partnership among three teams: E Gluckman from Hospital Saint Louis in Paris (France) who was working on the importance of attenuated dose conditioning regimens for FA patients (18); AD Auerbach from the Rockefeller University in New York (USA), who designed a method for prenatal diagnosis in FA (19); HE Broxmeyer from Indiana University in Indianapolis (USA), who studied hematopoietic progenitors in CB (20). Thus, the first CBT was performed at Saint Louis Hospital of Paris on a 5-year-old boy diagnosed with a BMF secondary to FA (17). Graft consisted of cryopreserved CB cells from the unaffected HLA-identical sister. The patient, conditioned with low-dose cyclophosphamide (Cy) and limited-field thoraco-abdominal irradiation, developed no major complication after CBT and reconstituted a month later with complete donor chimerism, maintaining a full immunological and hematological reconstitution 30 years after CBT (21,22). This was the first proof of concept that the CB of a single newborn is sufficient to reconstitute the host lympho-hematopoietic compartment definitely. After this first success, CB banks (CBB) arose all around the world for the gathering and cryopreservation of CB for allogeneic purposes (23).
The principal pragmatic advantages of using CB as stem cells source are the absence of risks for donors (and mothers), the reduced risk of transmitting infections, the relative prompt availability for immediate use (due to the ability to preserve fully tested and HLA-typed stem cell grafts in the frozen state). The Eurocord experience reported several studies focusing on outcomes of CBT in BMF since the late 1990s (24,25).
CBT in acquired severe AA (SAA)
Significant progress on the management of SAA has largely improved outcomes of patients who failed or relapsed after IST.
In young patients without MSD, current recommendations are to perform HSCT after the failure of one course of IST, if a fully matched unrelated donor (MUD) is available (26-29). In adults, an alternative donor HSCT is a considerable option as second-line therapy, for patients who fail one or two courses of IST (24,28).
Unlikely, for many patients, especially those from minority ethnic groups or more heterogeneous populations, it is impossible to identify a suitable BM unrelated donor. The possibility of an unrelated CBT (UCBT), as alternative graft option, has been thrivingly explored in patients with hematologic malignancies (30-35). However, only a few series are reported for SAA and other BMF. In primary reports incidence of graft failure was incredibly high and survival outcomes extremely poor (25), whereas since 2000 successful UCBTs for SAA have been reported only by few small series and case reports (36-38).
In 2008 the Japanese group reported on a cohort of 31 patients with a 2-year overall survival (OS) of 41% (39).
In a retrospective analysis from Eurocord on 71 patients diagnosed with SAA [9 with paroxysmal nocturnal hemoglobinuria (PNH)] who received a single UCBT (n=57, 80%) or double UCBT (n=14, 20%) the 3-year OS was 37% and 43% after double UCBT (40). In multivariate analysis, the only factor influencing engraftment and survival was pre-freezing total nucleated cell (TNC) dose (>3.9×107/kg, P=0.05).
A more recent survey of the Japan Society for Hematopoietic Cell Transplantation compared results of UCBT (n=69) to 8/8 (n=101), 7/8 (n=65) or 6/8 (n=37) -matched unrelated bone marrow transplantation (UBMT) from 2002 to 2012 (41). This study showed similar survival rates for adults less than 40 years of age in each of four groups and worst results for UCBT in patients older than 40 (47%, 64%, 64%and 75% of 3-year OS for UCBT, 8/8, 7/8 and 6/8 UBMT respectively), suggesting that the choice of UCBT for older adults should cautiously be considered in case of lacking an 8/8, or 7/8 matched adult donor. Those studies justify the use of double UCBT if necessary in the setting of SAA. However, graft failure remains a major concern in this particularly high-risk population and it is highly recommended to achieve the adequate cell dose threshold when considering CB units.
Results of a prospective phase II study (NCT01343953, APCORD Trial), evaluating the efficacy and safety of UCBT in refractory SAA patients, have been recently published on behalf of Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC) (42). Twenty-six patients were analyzed (out of 29 included). The conditioning regimen consisted of fludarabine (Flu) 30 mg/m2 from day −6 to day −3, Cy 30 mg/kg from day −6 to day −3, anti-thymocyte globulin (ATG) 2.5 mg/kg from day −3 to day −2, 2-Gray total body irradiation (TBI) on day −2. An injection of anti-CD20 at the dose of 150 mg/m2 was given at day +5 for prophylaxis of Epstein Barr virus (EBV) reactivation. Graft versus host disease (GVHD) prophylaxis was performed with cyclosporine A (CsA) alone. The median age at CBT was 16 years [interquartile range (IQR), 9.3–23.4 years]. All patients received at least 1 course of IST before transplantation (2 courses, n=5–11) with a median time between diagnosis and transplantation of (12 months; IQR, 8.7–17.8 months). Median follow-up was 38.8 months (IQR, 29.9–53.8 months). One-year survival rate was 88.5% [95% confidence interval (CI), 69.8–97.6%]. One-year treatment-related mortality was 11.5% (95% CI, 2.4–30.2%). Three patients died before 1 year due to infections arising from non-engraftment (n=2) and GVHD (n=1), and a further patient died of severe chronic GVHD at 13.9 months, leading to a 2-year survival rate of 84.6% (95% CI, 71–100%).
The graft failure and the unacceptable risk of severe infections have been major pitfalls of UCBT in refractory SAA patients (39,40). The key role of TNC doses (>3.9×107/kg) in umbilical transplants as well as the use of a Flu/low-dose TBI-based conditioning regimen to improve engraftment and survival outcomes, was suggested by retrospective studies (40), The APCORD study prospectively confirmed the importance of controlling these factors (42).
For patients with SAA, CBT from an unrelated donor should be considered only in the setting of clinical trials, when a suitable BM donor is not available and after the failure of IST. To avoid the risk of graft failure due to an allogeneic immunization, donor-specific antibodies should be screened before transplantation (43-45). One or two CB units may be used in SAA to reach at least 4×107 cryopreserved nucleated cells/kg with less than 2 of 6 HLA mismatches between the unit and the patient. Furthermore, particular attention should be paid to patient cytomegalovirus (CMV) status since CMV seronegativity is generally easier to manage. The importance of preventing infections and the availability of high-quality supportive care platforms are key elements for the success of this type of procedure, which should only be carried out in experienced centers.
CBT in inherited BMF syndromes
FA
FA is a rare inherited disorder characterized by congenital abnormalities and genomic instability responsible for progressive BMF, predisposition to solid and hematological malignancies, including acute myeloid leukemia (AML), myelodysplastic syndrome (MDS) and squamous cell carcinoma (SCC) (46,47). Although phenotypically different, most FA patients develop hematologic abnormalities within the natural history of their disease. Those abnormalities may range from mild hematologic changes not requiring therapeutic intervention, to the development of severe BMF or myeloid neoplasia, needing HSCT to restore the normal hematopoiesis (46). During the last decades, improvements of FA-HSCT conditioning regimens and advances in transplant procedures (e.g., more accurate HLA typing, graft manipulation, better supportive care, etc.) have drastically ameliorated the prognosis of FA patients (48,49). Reduction of radiation and chemotherapy doses, with the ideation of new reduced-intensity conditioning regimens (46,48), incorporation of Flu to preparative regimens (49,50), in vivo and/or ex vivo T cell depletion of allografts (51), were able to decrease the treatment-related mortality enhancing the engraftment and improving survival (48).
European Group for Blood and Marrow Transplantation (EBMT) retrospectively analyzed results of UCBT in 93 FA patients transplanted worldwide between 1994 and 2005 (14). The median age at transplantation was 8.6 years. The majority of patients received an HLA mismatched CBT (one mismatch in 35 cases and more than two mismatches in 45 cases). Sixty-one percent of patients received Flu within the conditioning regimen. The cumulative incidence function (CIF) neutrophil recovery was 60%±5% at day +60. The CIF of acute and chronic GVHD was 32.5% and 16%, respectively. The 2-year OS was 40%±5%. In the multivariate analysis Flu, a high number of TNC and negative recipient CMV serology were associated with favorable outcomes (14).
More recent reports, principally on small single-center experiences, show dismal results, namely high risk of graft failure, especially due to an incomplete HLA matching (52-54).
MacMillan et al. analyzed outcomes of 130 FA patients undergoing alternative donor HSCT (99 receiving BM and 31 receiving CB as graft source) (55). In this study conditioning regimens changed over the time, but since 2006, irrespective of graft source, all patients (48 out of 130) received TBI (300 cGy), Cy (10 mg/kg/day for 4 days), Flu (35 mg/m2/day for 4 days) horse ATG (30 mg/kg/day for 5 days), with CsA and mycophenolate mofetil (MMF) for GVHD prophylaxis. For the entire cohort, the probability of OS was 63% (95% CI, 54–71%) at 1 year and 58% (95% CI, 49–59%) at 5 years. The CIF of neutrophil recovery was 90% (95% CI, 84–95%) at day +30 and the use of CB was associated with a lower probability of engraftment compared with BM MUD, however, this outcome was strongly influenced by the type of conditioning regimen. For 46 recipients of the Flu/TBI 300 cGy-based conditioning regimen, neutrophil recovery was similar in recipients of BM and CB. The CIF of grade II–IV and grade III–IV acute GVHD was 20% (95% CI, 13–27%) and 9% (95% CI, 4–14%), with a similar likelihood for patients receiving a mismatched unrelated BM donor 7/8 HLA-matched T-cell-depleted BM and 4–6/6 HLA-matched CB.
To date, the outcomes of FA patients undergoing CBT versus BMT have not formally compared yet. However, the evidence is that the use of Flu is associated with better survival in spite of stem cell source (14,49), suggesting that this molecule acts as an immune suppressive agent, and enhances the engraftment without increasing extra-medullary toxicity.
In most reports, the use of CB unit with two or more HLA disparities in FA is associated with a lower probability of neutrophil recovery, decreased survival, or unacceptable rate of GVHD (14,52). For this reason, in this context, only one CB unit with no more than one mismatch is recommended (28).
Thus, UCBT, using a specific conditioning regimen disease-adapted, is indicated in FA patients who lack an HLA-matched unrelated BM donor. However, CB unit should be carefully selected, basing on HLA matching and TNC.
Inherited BMF other than FA
Until recently, outcomes of HSCT in other type of inherited BMF syndromes, such as in the context of DC, Shwachman-Diamond syndrome (SDS), DBA, etc. have been discouraging because of the high risk of transplant-related morbidity, including graft failure, GVHD, infectious complications and the propensity to develop organ toxicity (26,56-58).
In 2011, Eurocord reported on an analysis of 64 patients diagnosed with inherited BMF disorders other than FA receiving related (n=20) CBT and non-related (n=44) CBT (59). Diagnoses were DBA (21 patients), congenital amegakaryocytic thrombocytopenia (16 patients), DC (8 patients), SDS (2 patients), severe congenital neutropenia (16 patients) and unclassified (1 patient). The group who received the related CBT engrafted at day 60 in 95% of cases. The median number of TNC infused was 5×107/kg. Only two patients had grade II–IV acute GVHD, while the 2-year CIF of chronic GVHD was 11%, and the 3-year OS rate was 95%. In contrast, among patients who received grafts from unrelated donors, the CIF of neutrophil recovery was 55% at day 60 although the median number of infused TNC was 6.1×107/kg. Also, the 100-day CIF of grade II–IV acute GVHD was 24%, and the 2-year CIF of chronic GVHD was 53%, for a 3-year OS rate of 61%. In this group age less than 5 years (P=0.01) and more than 6.1×107/kg TNC (P=0.05) were factors associated with a better OS.
Generally speaking, HSCT from MSD remained the preferred choice, and very sporadic series are reported for each disease category to make any general recommendation. However, although retrospective, Eurocord studies provide evidence that in these particularly high-risk patients, related CBT can be associated with excellent results, while UCBT outcomes may be improved by an increasing of TNC dose and better HLA matching.
Specific situations: CBT from a matched related donor for children with BMF
Eurocord analysed, in partnership with Severe Aplastic Anemia Working Party (SAAWP) of the EBMT, the outcomes of 117 children and young adults diagnosed with acquired and inherited BMF, receiving a related HLA-identical CBT (60).
In this series, 82 patients received a single CB unit and 35 received a mixed graft (CB and BM cells from the same donor). The median age at transplantation was 6.7 years.
The CIF of neutrophil recovery (day 60) was 88.8% (95% CI, 83.1–94.9%) with a median time to engraftment of 21 days (range, 7–105 days). The 100-day CIF of acute grade II–IV GVHD was 15.2% (95% CI, 9.8–23.6%) and the 7-year CIF of chronic GVHD was 14.5% (95% CI, 8.6–24.2%).
With a median follow up of 7.2 years (1.5 months to 27.1 years), 7-year OS for the whole population was 87.9% (95% CI, 80.8–92.6%), 89% for inherited and 81% for acquired (P=0.66).
This study confirmed that CBT from an HLA-identical sibling donor could be a good option for patients with BMF since it is associated with excellent survival outcomes and low risk of GVHD and graft failure. In this setting collecting CB unit at the birth of a new sibling, especially in case of inherited BMF, should be strongly recommended.
Table 1 summarizes the main retrospective and prospective studies on CBT in BMF whereas Figure 1 shows principal survival outcomes.
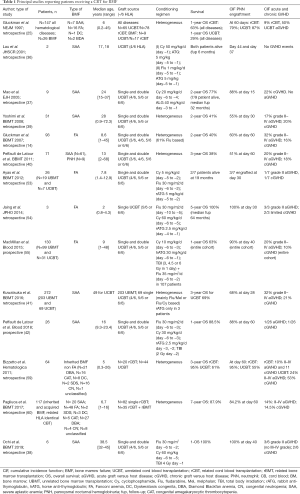
Full table

Emerging strategies and ongoing trials
The last EBMT activity survey, based on 2017 data and describing the status of HSCT in Europe and affiliated countries, reported on general decreasing use of CBT, mainly because of a growing number of haploidentical HSCT (61).
Of interest, in BMF, the whole number of patients receiving an allogeneic HSCT is slightly decreasing, and the number of CBT performed is very low (14 in 14 transplant centers). Such a change may be explained by the use of thrombopoietin (TPO) analogs in refractory AA patients. In spite of these changing trends in transplant activities a number of clinical trials, concerning the use of CB in patients diagnosed with BMF are ongoing and still recruiting patients, particularly in the setting of SAA (NCT02838992, NCT02745717, NCT00604201, NCT03173937, NCT01553461, NCT01586455).
Improving engraftment and promoting immune reconstitution are the main goals of new promising techniques, which are now entering clinical trials. Several strategies are in fact, object of study in order to increase the HSC dose of CB grafts.
Methods to enhance the homing to stem cell niches in the marrow have been variously investigated and still require extensive studies (62,63). In this setting, intra-bone infusion of CB cells may be beneficial in some contexts (64,65), even if this technique is far to be recommended.
Another evolving concept to promote CB engraftment is the expansion of HSCs by ex vivo or in vivo manipulations, concerning, for instance, the use of cytokines and growth factors in order to increase the progenitor compartment present in CB (66-68).
Earlier approaches concerned the use of culture media enriched with several cytokine combinations including TPO, granulocyte colony-stimulating factor (G-CSF), stem cell factor (SCF), erythropoietin (EPO), interleukin (IL)-3, IL-6, Fms-related tyrosine kinase 3 ligand (Flt-3L) (69-74). New strategies including insulin-like growth factor binding proteins, pleiotrophin, angiopoietin-like proteins, or novel combinations of mitogens are in the preclinical phase of study and need further refining before the development of clinical trials (75-77). Of interest, recently, a computerized modelling approach has been developed to select the optimal cytokine mix for the ex vivo expansion of CB stem cells (78).
The use of several molecules such as nicotinamide (Nicord), copper chelator tetraethylenepentamine (TEPA), UM729 (a pyrimidoindole derivative), StemRegenin-1 (SR1), Notch ligand, valproic acid or even carbon nanotubes are under development and demonstrated to inhibit HSC differentiation or promote their self-renewal (79-86).
The co-culture of CB cells with mesenchymal stem cells (MSCs) is a strategy whose basic principle is to simulate the physiological microenvironment of the BM (87,88), and co-transplantation of those two constituents has demonstrated to promote hematopoietic engraftment in patients undergoing UCBT transplantation (87,89).
Other approaches using genetically modified feeder cells are also under development (90).
Conclusions
In conclusion, in the absence of a suitable BM donor, CBT is an option for the treatment of patients with idiopathic and inherited BMF syndromes, especially if a sibling CB donor is used.
A better selection of the CB units (privileging units with more than 4×107 nucleated cells/kg) and an adaptation of the conditioning regimens can be able to overcome the risk of rejection.
In case of idiopathic context 1 or 2 CB units may be used with no more than 2 of 6 HLA mismatches between the unit and the recipient. In patients with inherited BMF, particularly in the setting of FA, the current recommendation is to choose a donor with no more than one HLA mismatch because of the risk of unacceptable toxicity,
For patients with FA and other inherited BMF only 1 CB is recommended with no more than one mismatch.
Donor specific antibody screening should be performed in every patient to minimize the risk of rejection. Ex-vivo CB expanding strategies aiming to better engraftment are under investigation.
When the cryopreserved CB unit from the HLA identical sibling does not contain enough cell dose, add-back of BM cells at the time of transplant, could be feasible with excellent results and no increase in GVHD (60,91).
A Flu-Cy-TBI-ATG conditioning regimen (APCORD trial) can be effective and safe in refractory SAA patients receiving a single or double UCBT (42), whereas limited data are available in inherited context, although retrospective evidence suggests to reduce chemotherapy and radiation doses, integrating Flu and T-depletion, especially in FA patients.
Organ toxicity remains problematic for most inherited BMF, and prospective international clinical trials are urgently needed to improve engraftment and GVHD-free survival.
Acknowledgments
The authors thank Eliane Gluckman and Eurocord members.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Sieff CA, Nisbet-Brown E, Nathan DG. Congenital bone marrow failure syndromes. Br J Haematol 2000;111:30-42. [Crossref] [PubMed]
- Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood 2012;120:1185-96. [Crossref] [PubMed]
- Young ME, Potter V, Kulasekararaj AG, et al. Haematopoietic stem cell transplantation for acquired aplastic anaemia. Curr Opin Hematol 2013;20:515-20. [Crossref] [PubMed]
- Gluckman E, Wagner JE. Hematopoietic stem cell transplantation in childhood inherited bone marrow failure syndrome. Bone Marrow Transplant 2008;41:127-32. [Crossref] [PubMed]
- Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev 2010;24:101-22. [Crossref] [PubMed]
- Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol 1988;70:177-82. [Crossref] [PubMed]
- Vlachos A, Muir E. How I treat Diamond-Blackfan anemia. Blood 2010;116:3715-23. [Crossref] [PubMed]
- Calado RT, Clé DV. Treatment of inherited bone marrow failure syndromes beyond transplantation. Hematology Am Soc Hematol Educ Program 2017;2017:96-101.
- Townsley DM, Dumitriu B, Liu D, et al. Danazol Treatment for Telomere Diseases. N Engl J Med 2016;374:1922-31. [Crossref] [PubMed]
- Rose SR, Kim MO, Korbee L, et al. Oxandrolone for the treatment of bone marrow failure in Fanconi anemia: Oxandrolone Use in FA Bone Marrow Failure. Pediatr Blood Cancer 2014;61:11-9. [Crossref] [PubMed]
- Khincha PP, Wentzensen IM, Giri N, et al. Response to androgen therapy in patients with dyskeratosis congenita. Br J Haematol 2014;165:349-57. [Crossref] [PubMed]
- Alter BP, Gardner FH, Hall RE. Treatment of dyskeratosis congenita with Granulocyte Colony‐Stimulating Factor and Erythropoietin. Br J Haematol 1997;97:309-11. [Crossref] [PubMed]
- Bacigalupo A, Oneto R, Bruno B, et al. Current results of bone marrow transplantation in patients with acquired severe aplastic anemia. Report of the European Group for Blood and Marrow transplantation. On behalf of the Working Party on Severe Aplastic Anemia of the European Group for Blood and Marrow Transplantation. Acta Haematol 2000;103:19-25. [Crossref] [PubMed]
- Gluckman E, Rocha V, Ionescu I, et al. Results of unrelated cord blood transplant in fanconi anemia patients: risk factor analysis for engraftment and survival. Biol Blood Marrow Transplant 2007;13:1073-82. [Crossref] [PubMed]
- Deeg HJ, O'Donnell M, Tolar J, et al. Optimization of conditioning for marrow transplantation from unrelated donors for patients with aplastic anemia after failure of immunosuppressive therapy. Blood 2006;108:1485-91. [Crossref] [PubMed]
- Maury S, Balère-Appert ML, Chir Z, et al. Unrelated stem cell transplantation for severe acquired aplastic anemia: improved outcome in the era of high-resolution HLA matching between donor and recipient. Haematologica 2007;92:589-96. [Crossref] [PubMed]
- Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med 1989;321:1174-8. [Crossref] [PubMed]
- Gluckman E. Radiosensitivity in Fanconi anemia: application to the conditioning for bone marrow transplantation. Radiother Oncol 1990;18 Suppl 1:88-93. [Crossref] [PubMed]
- Auerbach AD, Liu Q, Ghosh R, et al. Prenatal identification of potential donors for umbilical cord blood transplantation for Fanconi anemia. Transfusion 1990;30:682-7. [Crossref] [PubMed]
- Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A 1989;86:3828-32. [Crossref] [PubMed]
- Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood 2013;122:491-8. [Crossref] [PubMed]
- Wagner JE, Gluckman E. Umbilical cord blood transplantation: the first 20 years. Semin Hematol 2010;47:3-12. [Crossref] [PubMed]
- Gluckman E, Ruggeri A, Rocha V, et al. Family-directed umbilical cord blood banking. Haematologica 2011;96:1700-7. [Crossref] [PubMed]
- Bacigalupo A, Sica S. Alternative donor transplants for severe aplastic anemia: current experience. Semin Hematol 2016;53:115-9. [Crossref] [PubMed]
- Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med 1997;337:373-81. [Crossref] [PubMed]
- Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant 2010;16:S119-25. [Crossref] [PubMed]
- Kennedy-Nasser AA, Leung KS, Mahajan A, et al. Comparable outcomes of matched-related and alternative donor stem cell transplantation for pediatric severe aplastic anemia. Biol Blood Marrow Transplant 2006;12:1277-84. [Crossref] [PubMed]
- Peffault de Latour R. Transplantation for bone marrow failure: current issues. Hematology Am Soc Hematol Educ Program 2016;2016:90-8.
- Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of EBMT. Br J Haematol 2015;171:585-94. [Crossref] [PubMed]
- Sauter C, Barker JN. Unrelated donor umbilical cord blood transplantation for the treatment of hematologic malignancies. Curr Opin Hematol 2008;15:568-75. [Crossref] [PubMed]
- Ruggeri A, Volt F, Locatelli F, et al. Unrelated Cord Blood Transplantation for Acute Leukemia Diagnosed in the First Year of Life: Outcomes and Risk Factor Analysis. Biol Blood Marrow Transplant 2017;23:96-102. [Crossref] [PubMed]
- Eapen M, Kurtzberg J, Zhang MJ, et al. Umbilical Cord Blood Transplantation in Children with Acute Leukemia: Impact of Conditioning on Transplantation Outcomes. Biol Blood Marrow Transplant 2017;23:1714-21. [Crossref] [PubMed]
- Baron F, Ruggeri A, Beohou E, et al. Single- or double-unit UCBT following RIC in adults with AL: a report from Eurocord, the ALWP and the CTIWP of the EBMT. J Hematol Oncol 2017;10:128. [Crossref] [PubMed]
- Gerds AT, Woo Ahn K, Hu ZH, et al. Outcomes after Umbilical Cord Blood Transplantation for Myelodysplastic Syndromes. Biol Blood Marrow Transplant 2017;23:971-9. [Crossref] [PubMed]
- Robin M, Ruggeri A, Labopin M, et al. Comparison of unrelated cord blood and peripheral blood stem cell transplantation in adults with myelodysplastic syndrome after reduced-intensity conditioning regimen: a collaborative study from Eurocord (Cord blood Committee of Cellular Therapy & Immunobiology Working Party of EBMT) and Chronic Malignancies Working Party. Biol Blood Marrow Transplant 2015;21:489-95. [Crossref] [PubMed]
- Lau FY, Wong R, Chui CH, et al. Successful engraftment in two adult patients with severe aplastic anemia using nonmyeloablative conditioning followed by unrelated HLA-mismatched cord blood transplantation. J Hematother Stem Cell Res 2001;10:309-11. [Crossref] [PubMed]
- Mao P, Zhu Z, Wang H, et al. Sustained and stable hematopoietic donor-recipient mixed chimerism after unrelated cord blood transplantation for adult patients with severe aplastic anemia. Eur J Haematol 2005;75:430-5. [Crossref] [PubMed]
- Ochi T, Onishi Y, Nasu K, et al. Umbilical Cord Blood Transplantation Using Reduced-Intensity Conditioning without Antithymocyte Globulin in Adult Patients with Severe Aplastic Anemia. Biol Blood Marrow Transplant 2019;25:e55-9. [Crossref] [PubMed]
- Yoshimi A, Kojima S, Taniguchi S, et al. Unrelated cord blood transplantation for severe aplastic anemia. Biol Blood Marrow Transplant 2008;14:1057-63. [Crossref] [PubMed]
- Peffault de Latour R, Purtill D, Ruggeri A, et al. Influence of nucleated cell dose on overall survival of unrelated cord blood transplantation for patients with severe acquired aplastic anemia: a study by eurocord and the aplastic anemia working party of the European group for blood and marrow transplantation. Biol Blood Marrow Transplant 2011;17:78-85. [Crossref] [PubMed]
- Kuwatsuka Y, Kanda J, Yamazaki H, et al. A Comparison of Outcomes for Cord Blood Transplantation and Unrelated Bone Marrow Transplantation in Adult Aplastic Anemia. Biol Blood Marrow Transplant 2016;22:1836-43. [Crossref] [PubMed]
- Peffault de Latour R, Chevret S, Jubert C, et al. Unrelated cord blood transplantation in patients with idiopathic refractory severe aplastic anemia: a nationwide phase 2 study. Blood 2018;132:750-4. [Crossref] [PubMed]
- Yamamoto H, Uchida N, Matsuno N, et al. Anti-HLA antibodies other than against HLA-A, -B, -DRB1 adversely affect engraftment and nonrelapse mortality in HLA-mismatched single cord blood transplantation: possible implications of unrecognized donor-specific antibodies. Biol Blood Marrow Transplant 2014;20:1634-40. [Crossref] [PubMed]
- Morin-Zorman S, Loiseau P, Taupin JL, et al. Donor-Specific Anti-HLA Antibodies in Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol 2016;7:307. [Crossref] [PubMed]
- van Besien K. Advances in umbilical cord blood transplant: a summary of the 11th International Cord Blood Symposium, San Francisco, 6–8 June 2013. Leuk Lymphoma 2014;55:1735-8. [Crossref] [PubMed]
- Kutler DI, Singh B, Satagopan J, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR). Blood 2003;101:1249-56. [Crossref] [PubMed]
- Fanconi G. Familial constitutional panmyelocytopathy, Fanconi’s anemia (F.A.). I. Clinical aspects. Semin Hematol 1967;4:233-40. [PubMed]
- Eyrich M, Winkler B, Schlegel P. Stem Cell Transplantation in Fanconi Anemia – Recent Advances with Alternative Donors. In: Schindler D, Hoehn, H. editors. Fanconi Anemia. A Paradigmatic Disease for the Understanding of Cancer and Aging. Monogr Hum Genet. Basel: Karger, 2007:173-82. doi: 10.1159/000102555. [Crossref]
- Kapelushnik J, Or R, Slavin S, et al. A fludarabine-based protocol for bone marrow transplantation in Fanconi’s anemia. Bone Marrow Transplant 1997;20:1109-10. [Crossref] [PubMed]
- Bitan M, Or R, Shapira MY, et al. Fludarabine-based reduced intensity conditioning for stem cell transplantation of Fanconi anemia patients from fully matched related and unrelated donors. Biol Blood Marrow Transplant 2006;12:712-8. [Crossref] [PubMed]
- Drobyski WR. Evolving strategies to address adverse transplant outcomes associated with T cell depletion. J Hematother Stem Cell Res 2000;9:327-37. [Crossref] [PubMed]
- Ruggeri A, de Latour RP, Rocha V, et al. Double cord blood transplantation in patients with high risk bone marrow failure syndromes. Br J Haematol 2008;143:404-8. [Crossref] [PubMed]
- Ayas M, Al-Seraihi A, El-Solh H, et al. The Saudi experience in fludarabine-based conditioning regimens in patients with Fanconi anemia undergoing stem cell transplantation: excellent outcome in recipients of matched related stem cells but not in recipients of unrelated cord blood stem cells. Biol Blood Marrow Transplant 2012;18:627-32. [Crossref] [PubMed]
- Jaing TH, Chen SH, Yang CP, et al. Successful hematopoietic reconstitution by unrelated donor cord blood transplantation in children with Fanconi anemia: report of 3 cases. J Pediatr Hematol Oncol 2014;36:e553-5. [Crossref] [PubMed]
- MacMillan ML, DeFor TE, Young JA, et al. Alternative donor hematopoietic cell transplantation for Fanconi anemia. Blood 2015;125:3798-804. [Crossref] [PubMed]
- Barbaro P, Vedi A. Survival after Hematopoietic Stem Cell Transplant in Patients with Dyskeratosis Congenita: Systematic Review of the Literature. Biol Blood Marrow Transplant 2016;22:1152-8. [Crossref] [PubMed]
- Roy V, Pérez WS, Eapen M, et al. Bone marrow transplantation for diamond-blackfan anemia. Biol Blood Marrow Transplant 2005;11:600-8. [Crossref] [PubMed]
- Donadieu J, Michel G, Merlin E, et al. Hematopoietic stem cell transplantation for Shwachman-Diamond syndrome: experience of the French neutropenia registry. Bone Marrow Transplant 2005;36:787-92. [Crossref] [PubMed]
- Bizzetto R, Bonfim C, Rocha V, et al. Outcomes after related and unrelated umbilical cord blood transplantation for hereditary bone marrow failure syndromes other than Fanconi anemia. Haematologica 2011;96:134-41. [Crossref] [PubMed]
- Pagliuca S, Peffault de Latour R, Volt F, et al. Long-Term Outcomes of Cord Blood Transplantation from an HLA-Identical Sibling for Patients with Bone Marrow Failure Syndromes: A Report From Eurocord, Cord Blood Committee and Severe Aplastic Anemia Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 2017;23:1939-48. [Crossref] [PubMed]
- Passweg JR, Baldomero H, Basak GW, et al. The EBMT activity survey report 2017: a focus on allogeneic HCT for nonmalignant indications and on the use of non-HCT cell therapies. Bone Marrow Transplant 2019;54:1575-85. [Crossref] [PubMed]
- Cutler C, Multani P, Robbins D, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood 2013;122:3074-81. [Crossref] [PubMed]
- Brunstein CG, McKenna DH, DeFor TE, et al. Complement fragment 3a priming of umbilical cord blood progenitors: safety profile. Biol Blood Marrow Transplant 2013;19:1474-9. [Crossref] [PubMed]
- Frassoni F, Gualandi F, Podestà M, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: a phase I/II study. Lancet Oncol 2008;9:831-9. [Crossref] [PubMed]
- Brunstein CG, Barker JN, Weisdorf DJ, et al. Intra-BM injection to enhance engraftment after myeloablative umbilical cord blood transplantation with two partially HLA-matched units. Bone Marrow Transplant 2009;43:935-40. [Crossref] [PubMed]
- Zhang CC, Lodish HF. Cytokines regulating hematopoietic stem cell function. Curr Opin Hematol 2008;15:307-11. [Crossref] [PubMed]
- Tung SS, Parmar S, Robinson SN, et al. Ex vivo expansion of umbilical cord blood for transplantation. Best Pract Res Clin Haematol 2010;23:245-57. [Crossref] [PubMed]
- Chou S, Chu P, Hwang W, et al. Expansion of human cord blood hematopoietic stem cells for transplantation. Cell Stem Cell 2010;7:427-8. [Crossref] [PubMed]
- Mayani H, Dragowska W, Lansdorp PM. Cytokine-induced selective expansion and maturation of erythroid versus myeloid progenitors from purified cord blood precursor cells. Blood 1993;81:3252-8. [Crossref] [PubMed]
- Cicuttini FM, Welch KL, Boyd AW. The effect of cytokines on CD34+ Rh-123high and low progenitor cells from human umbilical cord blood. Exp Hematol 1994;22:1244-51. [PubMed]
- Mayani H, Lansdorp PM. Thy-1 expression is linked to functional properties of primitive hematopoietic progenitor cells from human umbilical cord blood. Blood 1994;83:2410-7. [Crossref] [PubMed]
- Piacibello W, Sanavio F, Garetto L, et al. Extensive amplification and self-renewal of human primitive hematopoietic stem cells from cord blood. Blood 1997;89:2644-53. [Crossref] [PubMed]
- Ohmizono Y, Sakabe H, Kimura T, et al. Thrombopoietin augments ex vivo expansion of human cord blood-derived hematopoietic progenitors in combination with stem cell factor and flt3 ligand. Leukemia 1997;11:524-30. [Crossref] [PubMed]
- De Felice L, Di Pucchio T, Mascolo MG, et al. Flt3LP3nduces the ex-vivo amplification of umbilical cord blood committed progenitors and early stem cells in short-term cultures. Br J Haematol 1999;106:133-41. [Crossref] [PubMed]
- Zhang CC, Kaba M, Ge G, et al. Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med 2006;12:240-5. [Crossref] [PubMed]
- Celebi B, Mantovani D, Pineault N. Insulin-like growth factor binding protein-2 and neurotrophin 3 synergize together to promote the expansion of hematopoietic cells ex vivo. Cytokine 2012;58:327-31. [Crossref] [PubMed]
- Himburg HA, Muramoto GG, Daher P, et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med 2010;16:475-82. [Crossref] [PubMed]
- Gullo F, van der Garde M, Russo G, et al. Computational modeling of the expansion of human cord blood CD133+ hematopoietic stem/progenitor cells with different cytokine combinations. Bioinformatics 2015;31:2514-22. [Crossref] [PubMed]
- Peled T, Mandel J, Goudsmid RN, et al. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy 2004;6:344-55. [Crossref] [PubMed]
- Peled T, Glukhman E, Hasson N, et al. Chelatable cellular copper modulates differentiation and self-renewal of cord blood-derived hematopoietic progenitor cells. Exp Hematol 2005;33:1092-100. [Crossref] [PubMed]
- Delaney C, Varnum-Finney B, Aoyama K, et al. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood 2005;106:2693-9. [Crossref] [PubMed]
- Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 2010;329:1345-8. [Crossref] [PubMed]
- Peled T, Shoham H, Aschengrau D, et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol 2012;40:342-55.e1. [Crossref] [PubMed]
- Chaurasia P, Gajzer DC, Schaniel C, et al. Epigenetic reprogramming induces the expansion of cord blood stem cells. J Clin Invest 2014;124:2378-95. [Crossref] [PubMed]
- Bari S, Chu PP, Lim A, et al. Mitochondrial superoxide reduction and cytokine secretion skewing by carbon nanotube scaffolds enhance ex vivo expansion of human cord blood hematopoietic progenitors. Nanomedicine 2015;11:1643-56. [Crossref] [PubMed]
- Angelos MG, Ruh PN, Webber BR, et al. Aryl hydrocarbon receptor inhibition promotes hematolymphoid development from human pluripotent stem cells. Blood 2017;129:3428-39. [Crossref] [PubMed]
- Wu KH, Sheu JN, Wu HP, et al. Cotransplantation of umbilical cord-derived mesenchymal stem cells promote hematopoietic engraftment in cord blood transplantation: a pilot study. Transplantation. 2013;95:773-7. [Crossref] [PubMed]
- de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med 2012;367:2305-15. [Crossref] [PubMed]
- Lee SH, Lee MW, Yoo KH, et al. Co-transplantation of third-party umbilical cord blood-derived MSCs promotes engraftment in children undergoing unrelated umbilical cord blood transplantation. Bone Marrow Transplant 2013;48:1040-5. [Crossref] [PubMed]
- Khan M, Ali I, Jiao W, et al. Ex vivo expansion of functional human UCB-HSCs/HPCs by coculture with AFT024-hkirre cells. Biomed Res Int 2014;2014:412075.
- Tucunduva L, Volt F, Cunha R, et al. Combined cord blood and bone marrow transplantation from the same human leucocyte antigen-identical sibling donor for children with malignant and non-malignant diseases. Br J Haematol 2015;169:103-10. [Crossref] [PubMed]
Cite this article as: Pagliuca S, Ruggeri A, Peffault de Latour R. Cord blood transplantation for bone marrow failure syndromes: state of art. Stem Cell Investig 2019;6:39.


