
Effect of selenium on freezing-thawing damage of mice spermatogonial stem cell: a model to preserve fertility in childhood cancers
Introduction
Background
In mankind the origin of germ cells is primordial germ cells (PGC) in both genders (1). PGCs are developed by bone morphogenic protein (BMP) signaling (such as BMP-4) (2). In male PGCs are surrounded by Sertoli cells in genital crest and seminiferous cords are created. Then PGCs are converted to gonocytes. These cells proliferate until arrest phase. After birth (in mice), they migrate lumen toward basal membrane of seminiferous cords and create spermatogonium-A and spermatogonium-B, and then back toward the lumen. One level of spermatogonium-A formation is called spermatogonial stem cell (SSC). These stem cell have self-renewal potency and therefore they can be stored for fertility preservation in future (3).
Infertility is a challenge of nowadays medicine. Male related infertilities are investigated using sperm analysis due to availability of sperm; but human oocytes are not easily available. Many sperm studies have shown that chemotherapy and radiotherapy can affect quality of sperm both in testicular cancers (4) and in other cancers (5). For instance, acute lymphoblastic leukemia (ALL) is common cancer in childhood. Poganitsch-Korhonen et al. mentioned that spermatogonial quantity decreased in treatment of ALL, based on biopsy studies (6). Therefore, a solution for fertility preservation in such conditions is storing frozen SSCs in prepubertal boys. Since at such ages puberty has not occurred, there is no sperm for preservation. In addition, SSCs have further potencies. Therefore, in such cases SSCs should be regarded for freezing instead of sperms. The process of freezing can be slow (cryopreservation) or rapid (vitrification) (3,7).
Many in vitro and in vivo studies have done both for animal modeling of testicular damage and for effects of different interventions in such conditions. These models are based on induction of cytotoxicity, oxidative stress [induction of reactive oxygen species (ROS)] (8) as well as induction of apoptosis (9). For example, for in vivo models, testicular torsion-detorsion for induction of ROS (10,11), gentamycin toxicity (12), or cisplatin induced testicular damage (13), and for in vitro models freezing-thawing damage (8) can be mentioned. The interventions include melatonin (14), ghrelin (15), selenium (16), medical plants (17) and so on.
Rationale
Childhood cancers requires chemotherapy. During cancer treatment, fertility of boys may be affected. Therefore, freezing SSC was recommended. However, freezing-thawing process may cause damage to SSCs. Hence adjuvant interventions should be carried out to reduce this damage. Investigation of this evidence gap requires animal model.
Objectives
Due to the importance of reduction of freezing-thawing damage, this study is conducted to evaluate protective effects of selenium on freezing-thawing damage of immature mice SSCs using investigation of cell viability and investigation of apoptosis related genes expression. These genes were Fas, Caspase3, Bcl2, Bax and P53. The practical aim of this study was to design an animal model of fertility preservation in order to use human research in childhood cancers.
Methods
Study design
This work was an in vitro experimental study consisting of testis resection from animals, SSC extraction from the resected testes, freezing-thawing process of the SSCs and then intervention.
Animals and study groups
A total of 80 6-day-old male BALB/c mice was used for resection of testis. Parents of the animals were kept in standard condition including 12-hour lightness and 12-hour darkness, free available water, free available nutritional concentrate and 20±2 °C of temperature. Birthday of the animals was considered as day 0. At day 6, the testes were resected.
SSC extraction was done using magnetic activated cell storing (MACS) technic as reported previously (18). The SSCs were divided into four groups: cryopreservation with selenium intervention (5 and 50 µg/mL), vitrification with selenium intervention (5 and 50 µg/mL), cryopreservation control group (without selenium), and vitrification control group (without selenium). Five µg/mL was regarded as low dose and 50 µg/mL was regarded as high dose.
Laboratory investigations
Testis resection
High-dose ketamine (80 mg/kg) and xylazine (10 mg/kg) were used for anesthesia. Suprapubic incision was performed and after finding urinary bladder, testes were found insides the urinary bladder. Under guide of dissection microscope, the testes were dissected and resected at sterile condition.
Cellular extraction
The testes were put in a Dulbecco’s modification of Eagle medium (DMEM) containing penicillin/streptomycin 10%. Tunica albuginea of the testes were removed under dissection microscope. Seminiferous tubes were transferred to a cone tube containing DMEM, 2 mg/mL collagenase and 200–500 µg/mL DNaseI. The tubes were shaken for 15 minutes at 37 °C. The tubes were washed with DMEM for 2 times. An amount of 1 mL trypsin/EDTA was added to the pallet resulted from previous step; then the mixture was pipetted and shaken in order to separate out the cells (200–500 µg/mL DNaseI was also added). The cellular suspension was filtered with 60-micron nylon mesh (Figure 1).
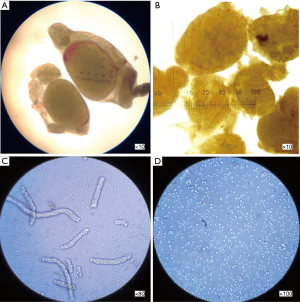
SSC extraction
MACS technic was used for extraction of SSCs. SSCs were extracted using antibody-linked microbeads according to guideline of the manufacture (Miltenyi Biotec, Germany). The complete procedure was described previously (19).
Cell viability
Trypan blue staining was used to count live cells. Neobar slide was used to count stain negative cells under light microscope. Trypan blue positive cells were considered as dead cells.
Freezing process
For cryopreservation, cell containing cryovials were maintained for 20 minutes at 4 °C, 1 hour at −20 °C and 1 day at −70 °C. Then they were stored in liquid azote tank (−196 °C). For vitrification, the cryovials were kept in nitrogen vapor for seconds, and then they were transferred to liquid azote tank.
Thawing process
The vials were removed from azote and put on dry ice. Then below half of the vials were put in 37 °C bain-marie for 2–3 minutes. A new culture environment was added to the vials. Then the mixtures were transferred to a falcon containing cell culture environment in 37 °C.
Gene expression study
Column-based method was used for extraction of RNA. Total RNA purification kit (Jena Bioscience, Germany) was used according to guideline of the manufacture. All equipment were DNase and RNase free. For cDNA synthesis a random hexamer-based kit (Bioneer, South Korea) was used according to guideline of the manufacture. Real-time PCR was used to investigate gene expression. The primers were designed using NCBI and Primer 3 software (Bioinfo, Estonia). GAPDH was used as internal control. The primers are shown (Table 1). SYBER green master mix was used (Jena Bioscience, Germany). Electrophoresis gel was used to validate the sizes of real-time PCR products. Relative expressions were reported using Rest 2009 software (Technical University, Munich).

Full table
Ethical considerations
This work was done according to ethical guidelines of working with laboratory animals under supervision of the ethics committee of Lorestan University of Medical Sciences LUMS.REC.1395.162.
Statistical analysis
One-way ANOVA was used to investigate significance of differences among the groups of study using SPSS version 21 software (IBM, US). Standard error of means (SEM) was reported as error bar. Gene expression analysis was done using Rest 2009 software. P=0.05 was considered as significant level.
Results
Viability study
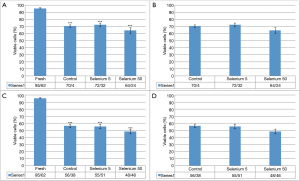
Frequency of viable cells in fresh samples (without freezing-thawing process) was 95.62%. The frequency in cryopreservation control group, cryopreservation group with selenium 5 µg/mL and cryopreservation group with selenium 50 µg/mL group were 70.40%, 72.32% and 64.34%, respectively. The reduction of cell viability in these three groups in comparison to the group of fresh samples was significant (P<0.001). Comparison of cell viability these three groups did not show a significant association. Frequency of viable cells in vitrification control group, vitrification group with selenium 5 µg/mL and vitrification group with selenium 50 µg/mL group were 65.38%, 55.51% and 48.46%, respectively. The reduction of cell viability in these three groups in comparison to the group of fresh samples was significant (P<0.001). Comparison of cell viability these three groups did not show a significant association. All analyses were done with analysis of variance (ANOVA) (Figure 2).
Gene expression study
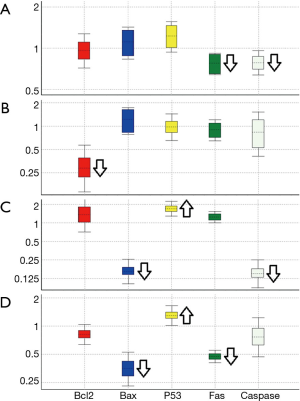
Expression of Bcl2, Bax and P53 was not statistically different between cryopreservation control group and the cryopreservation group administered with selenium 5 µg/mL. However, relative expression of Fas and Caspase3 was significantly lower in the intervention group (P<0.001). Expression of Fas, Caspase3, Bax and P53 was not statistically different between cryopreservation control group and the cryopreservation group administered with selenium 50 µg/mL. However, relative expression of Bcl2 was significantly lower in the intervention group (P<0.001). Expression of Bcl2 and Fas was not statistically different between vitrification control group and the vitrification group administered with selenium 5 µg/mL. However, relative expression of Bax and Caspase3 was significantly lower in the intervention group, and relative expression of P53 was significantly upper (P<0.001). Expression of Bcl2 and Caspase3 was not statistically different between vitrification control group and the vitrification group administered with selenium 50 µg/mL. However, relative expression of Bax and Fas was significantly lower in the intervention group, and relative expression of P53 was significantly upper (P<0.001) (Table 2, Figure 3).
Full table
Discussion
The present study was aimed to investigate the effects of administration of selenium on freezing-thawing damage of SSCs. This damage was investigated using cellular viability and expression of apoptosis related genes. In this regard, trypan blue staining and real-time polymerase chain reaction (real-time PCR) were performed, respectively.
The results of trypan blue staining showed a significant reduction in cell viability for all study groups in comparison to fresh cells. This result showed that this in vitro modeling of cellular damage was performed correctly. The results of real-time PCR showed a dose-dependent effect for selenium on expression profile of apoptosis related genes. Briefly, selenium in both doses resulted in up regulation of P53 and down regulation of Bax in vitrification intervention group, as well resulted in down regulation of Caspase3 in low-dose intervention both in cryopreservation and vitrification intervention groups. Down regulation of Bcl2 occurred only in high-dose intervention of selenium in cryopreservation group. Down regulation of Fas occurred in low-dose intervention of selenium in cryopreservation group and high-dose intervention of selenium in vitrification group.
Among the apoptosis related proteins, Bcl2 is inhibitor of apoptosis whereas Fas, Bax, P53 and Caspase3 are the activators. Among them, Fas is for extrinsic pathway while P53 may affect both pathways. Of course, Caspase3 can be considered as a common pathway (20,21). Our study showed different effects of selenium on these pathways in different doses and different conditions according to real-time PCR study. Low-dose selenium in cryopreservation may inhibit apoptosis via inhibition of extrinsic pathway; however, this inhibition was not significant according trypan blue staining. High-dose selenium inhibits Bcl2 in cryopreservation; however, the outcome on apoptosis is not clear. In vitrification condition both low and high doses of selenium up regulate P53 and down regulate Bax (in low dose Caspase3 is down regulated and in high dose Fas is down regulated); however, the outcome on apoptosis is not clear, because the effect on P53 and Bax is contradictory. The scheme of these findings is shown (Figure 4).

History of investigation of the effects of selenium on spermatogenesis and male reproduction system backs to the previous century. Saaranen et al. (1989) investigated selenium content of semen on mitochondrial sheath of sperms in human and animals. They found no significant correlation in human (22). Presence of selenium in semen give researchers this rationale to use selenium in in vitro studies. Younis et al. (1998) investigated the effects of insulin transferrin selenium (ITS) combination on cryopreservation of Chimpanzee spermatozoa and they found better sperm motility. In contrast, our study was on SSCs instead of spermatozoa (23). Wu et al. (2009) performed a study on cryopreservation of rat SSCs. They used a combination which one of its components was selenium. Investigation of gene study had been suggested (24). Many other studies had investigated the effects of ITS on cryopreservation; however, there was no study on using selenium alone on freezing-thawing damage based on gene expression study. Rezaeian et al. (2016) investigated the effects of selenium on human sperm parameters in frozen-thawed samples. They showed that low-dose selenium administration could increase sperm motility (25). Pourmasumi et al. (2018) conducted a clinical trial. They found that combined supplementation of vitamin E and selenium sperm parameters (26). Ghafarizadeh et al. (2018) investigated effects selenium supplementation on sperm quality of asthenoteratozoospermic men. They found that in vitro selenium supplementation might protect sperms from ROS (27). Recently (2019), effects of selenium nanoparticles have been investigated in bull sperm cryopreservation and they found positive results (28).
Many in vivo studies have shown effects of selenium on animal oxidative stress models. Kaur and Bansal (2015) investigated effects of selenium on mice scrotal hyperthermia induced oxidative stress and they found protective effects via antioxidant and anti-apoptotic effects (16). Kara et al. (2016) showed protective effects of selenium on testicular ischemia-reperfusion injury of rat via antioxidant and anti-apoptotic effects (29). Kaur et al. in another study (2018) showed that selenium had antioxidant and anti-apoptotic effects in bisphenol A induced oxidative stress model of mice (30).
Briefly, selenium is an important element in semen and male fertility. In vivo studies showed protective effects of selenium on different animal models of testicular injury. In vitro studies showed that selenium and selenium containing compounds had protective effects on the sperms undergone freezing-thawing damage. In contrast, in the present study, selenium could not increase cellular viability of the SSCs undergone freezing-thawing damage. However, selenium could influent apoptosis related genes expression profile. Since SSC preservation process in children with cancer is an in vitro process, the evidence of using the results in clinics should be based on such studies.
Limitations
Lack of significant association in cellular viability analysis may be due to low power of the design of our study. Lack of cell culture timeline can be pointed out; because gene expression merely shows behavioral tendencies of cells, and it takes time to show clinical manifestation.
Conclusions
Administration of selenium could not increase cellular viability of SSCs after freezing-thawing damage of cryopreservation and vitrification procedures. According to gene expression study, selenium had dose-dependent effect on apoptosis related genes profile. The only evident effect was the effect of low-dose selenium in cryopreservation on inhibition of apoptosis via extrinsic pathway. Therefore, in vitro administration of selenium to SSCs of the children undergone cancer treatment may not be clinically beneficial based on the current evidence.
Acknowledgments
This study has supported by Lorestan University of Medical Sciences.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This work was done according to ethical guidelines of working with laboratory animals under supervision of the ethics committee of Lorestan University of Medical Sciences (IR.LUMS.REC.1395.162).
References
- Wu J, Zheng Z, Wang H, et al. Primordial germ cells and germ line stem cells. In: Zhao R. editor. Stem Cells: Basics and Clinical Translation. Dordrecht: Springer; 2015:3-28.
- Donoughe S, Nakamura T, Ewen-Campen B, et al. BMP signaling is required for the generation of primordial germ cells in an insect. Proc Natl Acad Sci U S A 2014;111:4133-8. [Crossref] [PubMed]
- Gholami M, Ahmadi SAY, Abaszadeh A, et al. Protective effects of melatonin and ghrelin on spermatogenesis: A narrative review of the literature. Int J Reprod Biomed (Yazd) 2017;15:265-72. [Crossref] [PubMed]
- Ghezzi M, Berretta M, Bottacin A, et al. Impact of BEP or carboplatin chemotherapy on testicular function and sperm nucleus of subjects with testicular germ cell tumor. Front Pharmacol 2016;7:122. [Crossref] [PubMed]
- Paoli D, Rizzo F, Fiore G, et al. Spermatogenesis in Hodgkin's lymphoma patients: a retrospective study of semen quality before and after different chemotherapy regimens. Hum Reprod 2016;31:263-72. [PubMed]
- Poganitsch-Korhonen M, Masliukaite I, Nurmio M, et al. Decreased spermatogonial quantity in prepubertal boys with leukaemia treated with alkylating agents. Leukemia 2017;31:1460. [Crossref] [PubMed]
- Aliakbari F, Yazdekhasti H, Abbasi M, et al. Advances in cryopreservation of spermatogonial stem cells and restoration of male fertility. Microsc Res Tech 2016;79:122-9. [Crossref] [PubMed]
- Bansal AK, Bilaspuri GS. Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int 2010. [Crossref] [PubMed]
- Hajiaghalou S, Ebrahimi B, Shahverdi A, et al. Comparison of apoptosis pathway following the use of two protocols for vitrification of immature mouse testicular tissue. Theriogenology 2016;86:2073-82. [Crossref] [PubMed]
- Kheirollahi A, Abbaszadeh A, Anbari K, et al. Troxerutin protect sperm, seminiferous epithelium and pituitary-gonadal axis from torsion-detorsion injury: An experimental study. Int J Reprod Biomed (Yazd) 2018;16:315-22. [Crossref] [PubMed]
- Shokoohi M, Madarek EO, Khaki A, et al. Investigating the Effects of Onion Juice on Male Fertility Factors and Pregnancy Rate After Testicular Torsion/Detorsion by Intrauterine Insemination Method. International journal of women's health and reproduction sciences 2018;6:499-505. [Crossref]
- Aly HAA, Hassan MH. Potential testicular toxicity of gentamicin in adult rats. Biochem Biophys Res Commun 2018;497:362-7. [Crossref] [PubMed]
- Yan WH, Liu D, Lu HY, et al. Significance of tumour cell HLA-G5/-G6 isoform expression in discrimination for adenocarcinoma from squamous cell carcinoma in lung cancer patients. J Cell Mol Med 2015;19:778-85. [Crossref] [PubMed]
- Li B, He X, Zhuang M, et al. Melatonin Ameliorates Busulfan-Induced Spermatogonial Stem Cell Oxidative Apoptosis in Mouse Testes. Antioxid Redox Signal 2018;28:385-400. [Crossref] [PubMed]
- Salimnejad R, Rad JS, Nejad DM. Protective Effect of Ghrelin on Oxidative Stress and Tissue Damages of Mice Testes Followed by Chemotherapy With Cyclophosphamide. Crescent J Med Biol Sci 2018;5:138-43.
- Kaur S, Bansal MP. Protective role of dietary-supplemented selenium and vitamin E in heat-induced apoptosis and oxidative stress in mice testes. Andrologia 2015;47:1109-19. [Crossref] [PubMed]
- Zhao WH, Zhai H, Wang L, et al. The protective effects of tea polysaccharides on injury and apoptosis of mouse sertoly cells induced by glyphosate. Curr Top Nutraceutical Res 2016;14:81-9.
- Valli H, Sukhwani M, Dovey SL, et al. Fluorescence- and magnetic-activated cell sorting strategies to isolate and enrich human spermatogonial stem cells. Fertil Steril 2014;102:566-580.e7. [Crossref] [PubMed]
- Korkusuz P, Köse S, Yersal N, et al. Magnetic-Based Cell Isolation Technique for the Selection of Stem Cells. Protocol. Available online: https://linkspringercom/protocol/101007/7651_2018_151 2018
- Krautwald S, Ziegler E, Rölver L, et al. Effective blockage of both the extrinsic and intrinsic pathways of apoptosis in mice by TAT-crmA. J Biol Chem 2010;285:19997-20005. [Crossref] [PubMed]
- Amaral JD, Xavier JM, Steer CJ, et al. The role of p53 in apoptosis. Discov Med 2010;9:145-52. [PubMed]
- Saaranen M, Suistomaa U, Vanha-Perttula T. Semen selenium content and sperm mitochondrial volume in human and some animal species. Hum Reprod 1989;4:304-8. [Crossref] [PubMed]
- Younis AI, Rooks B, Khan S, et al. The effects of antifreeze peptide III (AFP) and insulin transferrin selenium (ITS) on cryopreservation of chimpanzee (Pan troglodytes) spermatozoa. J Androl 1998;19:207-14. [PubMed]
- Wu Z, Falciatori I, Molyneux LA, et al. Spermatogonial culture medium: an effective and efficient nutrient mixture for culturing rat spermatogonial stem cells. Biol Reprod 2009;81:77-86. [Crossref] [PubMed]
- Rezaeian Z, Yazdekhasti H, Nasri S, et al. Effect of selenium on human sperm parameters after freezing and thawing procedures. Asian Pacific Journal of Reproduction 2016;5:462-6. [Crossref]
- Pourmasumi S, Ghasemi N, Talebi AR, et al. The Effect of Vitamin E and Selenium on Sperm Chromatin Quality in Couples with Recurrent Miscarriage. International Journal of Medical Laboratory 2018;5:1-10.
- Ghafarizadeh AA, Vaezi G, Shariatzadeh MA, et al. Effect of in vitro selenium supplementation on sperm quality in asthenoteratozoospermic men. Andrologia 2018. [Crossref] [PubMed]
- Khalil WA, El-Harairy MA, Zeidan AE, et al. Impact of selenium nano-particles in semen extender on bull sperm quality after cryopreservation. Theriogenology 2019;126:121-7. [Crossref] [PubMed]
- Kara Ö, Sari E, Akşit H, et al. Effects of selenium on ischaemia–reperfusion injury in a rat testis model. Andrologia 2016;48:1267-73. [Crossref] [PubMed]
- Kaur S, Saluja M, Bansal MP. Bisphenol A induced oxidative stress and apoptosis in mice testes: Modulation by selenium. Andrologia 2018. [Crossref] [PubMed]
Cite this article as: Boroujeni MB, Peidayesh F, Pirnia A, Boroujeni NB, Ahmadi SA, Gholami M. Effect of selenium on freezing-thawing damage of mice spermatogonial stem cell: a model to preserve fertility in childhood cancers. Stem Cell Investig 2019;6:36.


