
A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies
Introduction
Mesenchymal stem cells (MSCs)are hierarchical postnatal stem cells, capable of self-renewing and retain diverse differentiation potency into multi-lineages (1). MSCs turn out to be a prominent issue in latest research era, due to their biological significance and clinical applications. MSCs possess distinctive characteristics such as; ease of isolation and cultivation, plasticity, intrinsic tropism towards injured area (homing). They have also anti-inflammatory and anti-apoptotic activity in threatened tissues as well as immunomodulatory action by paracrine function, antimicrobial activity and bacterial clearance effect. They can activate other resident stem cells and stimulate neo-angiogenesis (2). These exceptional properties make MSCs an appropriate resource for the clinical treatment of some human diseases. As yet, cell therapy by MSCs has been effectively utilized for treating certain disorders, including metabolic, degenerative and inflammatory diseases, repair and regeneration of damaged or lost tissues on treatment of cancer (3). The current review briefly focuses upon promoting the perception of MSCs potentials, functions and clinical perspectives. Furthermore, the review addresses the evidence for how MSCs offers direction for further investigation and challenges of modification strategies.
What are MSCs and their potentials?
The mammalian bone marrow is responsible for hematopoiesis and bone homeostasis. It comprises a heterogeneous population of hematopoietic and non-hematopoietic stem cells such as fibroblast precursors, well known as MSCs. The International Society for Cellular Therapy (ISCT) has defined typical criteria for MSCs. MSCs must be plastic adherent and capable of differentiate to osteoblasts, adipocytes and chondroblasts lineages. They generally should express some unique surface antigens and not to express some others to meet the criteria (Table 1) (4-6). MSCs commonly exhibit low immunogenicity. They demonstrate simply intermediate expression levels of MHCI and no, or very low, expression of MHCII antigens and co-stimulatory molecules. Expression of MHCI prevented MSCs from acting like NK cells, while the lack of co-stimulatory molecules causes energy in T cells (3).

Full table
MSCs mechanisms of therapy
Migration (Homing)
“Homing” is the process of MSCs selective migration ability toward the site of injury and sustained delivery of the trophic signals. Expressing specific receptors or ligands by damaged tissues facilitate trafficking, adhesion, and infiltration of MSCs to the injured site. The process of MSC homing sequentially consists of three major steps. First, MSCs chemo attraction toward inflammation sites achieves by chemotaxis toward some accumulated chemokines and cytokines there, including EGF (epidermal growth factor), IGF (insulin like growth factor), PDGF (platelet-derived growth factor), VEGF (vascular endothelial growth factor), SDF-1 (stromal cell-derived factor 1), TNF-α (tumor necrosis factor α), IL-1, IL-6 and IL-8. Several potent stimulators like VCAM-1, MCP-1, MCP-3, G-CSF and hypoxia can arouse MSCs mobilization. Furthermore, SDF-1 (or CXCL12), TLRs and TNF- α induce the chemokine receptors (CXCR4 and CCR7) expression, which in turn boost up MSC chemo attraction. Second, MSCs adhesion to the injured cells attains by adhesion molecules such as Selectins and Integrins. Third, MSCs are infiltrated into inflammation sites by some enzymes such as MMPs (matrix metalloproteinase) and TIMPs (tissue inhibitors of matrix metalloproteinase) (7). When sites of injury are spreading or the damaged tissue is not easily accessible, the homing ability of MSCs is particularly valuable (8).
Tissue repair and regeneration
Some functional properties make MSCs appropriate for tissue regeneration and repair. These properties include MSCs capability to differentiate into several cell lineages, their homing capacity to migrate to injured tissues, angiogenesis, anti-apoptotic activity and finally their competency to secrete bioactive soluble factors. MSCs alter the tissue microenvironment through secretion of paracrine factors (4,9). Paracrine signaling significantly regulate proliferation, anti-oxidant activity and differentiation (10). It not only calls up macrophages and endothelial cells, but the signals also likely to stimulate resident stem cells to help the tissue repair process (3,11). Accordingly, the mechanism of regeneration is triggered by stimulation of endogenous repair programs via increasing proliferation of differentiated cells or activating of resident stem cells (12).
Immunomodulation
Most likely, several cytokines and regulatory factors attribute to immunomodulatory feature of MSCs. These factors including IL10, TGFβ, PGE2, IDO, NO, and FAS/FASL, probably act by inhibiting the proliferation and function of some immune cells such as B and T lymphocytes, dendritic cells, natural killer cells, monocytes, neutrophils, and macrophages (3). MSCs feasibly arrest B-cell proliferation, maturation, impair isotype-switching, inhibit chemotaxis, up-regulate antibody secretion (IgG), diminish pro-inflammatory cytokine secretion by Th1 cells, increase secretion of IL-4 by Th2 cells, inhibit T cells proliferation, increase formation of regulatory T cells and decrease cytotoxic effects of CTL. MSCs possibly suppress dendritic cells (DCs) differentiation, antigen presentation to T cells and inhibit the proliferation, activation and cytotoxic effects of natural killer (NK) cells. They can lessen local infiltration and activation of neutrophils, which release pro-inflammatory cytokines, enzymes and reactive oxygen species. They may possibly up-regulate genes responsible for phagocytosis in macrophages, so improve bacterial clearance and down-regulate inflammatory cytokine production by macrophages. Definite TLRs have main role in determining the immunosuppressive properties of MSCs. As such, MSCs can preserve peripheral tolerance in autoimmunity and any disorders (2,4).
Anti-inflammatory effects
Anti-inflammatory effects of MSCs protect the host by dampening the severity of immune response to inflammation. An overall reduction in both local and systemic inflammation undertakes by a balanced decrease of pro-inflammatory cytokine and increase of anti-inflammatory cytokine (7).
Anti-apoptotic activity
MSCs can protect injured cells and preserve organ function by inhibiting the programmed cell death through paracrine signaling. MSCs’ anti-apoptotic mechanisms include up-regulating DNA repair, down-regulating mitochondrial death pathways, increasing antioxidant activity and altering anti- and pro-apoptotic protein expression (2,7). Mediators secreted by MSCs include SDF-1, IGF-1, Nrf2, HIF, HO-1 and VEGF down regulate pro-apoptotic proteins (13-15).
Neoangiogenesis
MSCs can promote neovascularization in injured tissues by expression of angiogenic cytokines such as VEGF, FGF1, 2 (fibroblast growth factor), HGF (hepatocyte growth factor), Ang-1, 2 (angiopoietin), SDF-1. Secretion of soluble factors by MSCs make them capable of improving tissue vascularity by stimulating endothelial cell new growth and neoangiogenesis (2).
Activation of resident stem cells
Growth factors secreted by MSCs may be involved in the mobilization of resident stem cell populations. MSCs secreted VEGF (as a key mobilizer of stem cells), HGF and IGF-1 (insulin-like growth factor) to stimulate endogenous population of stem cell proliferation through complex paracrine and cell-to-cell interactions (2).
Antimicrobial Effects
MSCs are equipped with intrinsic bacterial killing mechanism by secreting the anti-microbial peptides such as LL-37 and Lipocalin-2 in response to stimulation by pathogens. MSC-derived antimicrobial factors most likely disrupt bacterial membranes and contribute to bacterial clearance (2). MSCs mechanisms of therapy have been summarized in Figure 1.
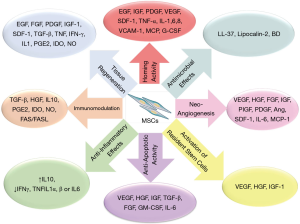
Therapeutic potential of MSCs
Due to the unique therapeutic properties of MSCs, there have been excessive interests in employing them for several therapies. MSCs have been proved to be effective in engraft in multiple organs, the repair of cardiovascular, lung and spinal cord injuries, autoimmune diseases, liver, bone and cartilage diseases (16).
Administration routes and effective dose of MSCs
Determining the success of MSC therapy somewhat depends on their potential administration subjected to local or systemic paracrine activity. There are consistently three possible routes for MSCs infusion, everyone has advantages and disadvantages. One route is systemic delivery [intra-venous (IV) and intra-arterial (IA) as well as inhalation]. The second is local/topical/regional delivery (cell-spray, gel or subcutaneous injection with a carrier hydrogel, intra-peritoneal (IP), intramuscular, or intra-cardiac (IC) and intra-thecal injection), and the third is scaffold/bioengineered construct (cells embedded in a scaffold, such as vascular grafts and intra-osseous injection), which is a kind of local delivery. In addition to routes of administration, the delivery of a number of sufficient MSCs as an effective dose (ED) requires to discern a significant therapeutic effect. However, a consensus dose of MSC has declared as 1×106/30 g mouse equal to 33×106/kg human, significantly lower MSC doses administrate in most humans clinical trials (17).
MSC transplantation (Local implantation and Systemic transplantation)
As MSCs are hypo-immunogenic, allogeneic MSCs transplantation can be considered safe. Delivering MSCs in the field of regenerative medicine has provided an attractive clinical treatment. In the main, the use of MSCs in local implantation for local tissue defects, systemic transplantation for generalized and systemic diseases and as a vehicle for gene delivery is prospective (18).
As a search term of “mesenchymal stem cells” on www.clinicaltrials.gov (January 2016) listed 577 trials. Again, the same term search on www.pubmed.com (January 2016) (with filters activated for Clinical Trial & Humans) listed 370 trials.
MSC transplantation in some diseases
Clinical applications of MSCs, for several diseases, have been registered at the National Institutes of Health ClinicalTrials.gov website (https://www.clinicaltrials.gov/)as clinical trials in different phases. The approximate percentages of trials while writing this review were about; cardiovascular diseases (15%), neuro degenerative diseases (12%), bone and cartilage diseases (6%), cancers (5%), liver diseases (3%), kidney diseases (3%), autoimmune diseases (25%) [including: graft versus-host diseases (GvHD, 9%), multiple sclerosis (MS, 4%), Crohn’s disease (3%), type1 diabetes (T1D, 5%), systemic lupus erythematous (SLE, 2%), rheumatoid arthritis (RA, 2%)] and many other diseases (31%) (Figure 2).
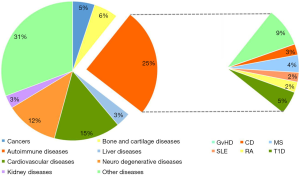
Liver diseases and MSC transplantation
However, MSCs have been utilized in a limited number of liver disease trials; therapeutic effects of MSCs for the treatment of liver diseases are promising (2). MSCs potential for liver diseases’ therapy relies on differentiation into hepatocytes besides immunomodulation by release of trophic factors affecting function of NK cells and stellate cells. As recently, MSC-dependent liver regeneration and immunomodulation has been comprehensively improved for treatment of both acute and chronic liver failure in some animal models (19). MSC therapy in liver disease is not only safe feasible and effective, but also is less invasive and have no drawbacks such as lack of donors, graft rejection, and surgical complications of liver transplantation (16). To date, 50 studies have been found for MSCs and liver diseases clinical trials (https://www.clinicaltrials.gov/).
Cardiovascular diseases and MSC transplantation
MSC therapy for cardiovascular diseases is promising, because it can repair and regenerate cardiac tissues besides immunomodulation. MSCs, besides homing into sites of myocardial damage, lacking both major MHCII and T-cell co-stimulatory signals, are immune privilege so the allogeneic MSCs are well tolerated (20). They contribute in cardiac regeneration not only by differentiating into cardio myocytes and vascular lineages but also through paracrine effects and secretion of a variety of angiogenic, mitogenic, anti-apoptotic factors and cocktail of growth factors (6,21). In preclinical models of heart disease as well as in clinical trials, using MSCs have exhibited improvement in cardiac repair (16,22). At the present almost 90 trials have registered inspecting the effect of MSC therapy in cardiac diseases (https://www.clinicaltrials.gov/).
Autoimmune diseases and MSC transplantation
As MSCs possess the capacity to modulate immune responses then maintain the peripheral tolerance, they are used to mitigate immune disorders as a safer and more practical method for control of autoimmunity (6). The therapeutic effect of MSCs has been scrutinized in patients with graft versus host disease (GvHD), Crohn’s disease (CD), multiple sclerosis, (MS) systemic lupus erythematous (SLE), rheumatoid arthritis (RA) and type1 diabetes (16).
Graft versus host disease (GvHD)
The clinical efficacy of MSCs to regulate tissue generation and repair in GvHD has been manifested. MSCs’ self-renewal, differentiation capacity and preventing Tcell proliferation in response to antigenic stimuli, along with anti-proliferation of B, natural killer and dendritic cells, make them suitable for immune-suppression (23,24).
Crohn’s disease
Pre-clinical studies proposed that immunomodulatory effects of MSCs would possibly ameliorate the pathogenesis of IBD. However, it would be rather MSC not to be administrated alone but along with antibodies and with genetic modification of autoimmune regulators would be more effective. Furthermore, it seems that administration routes of MSC is important to get better results (22).
Multiple sclerosis (MS)
MSCs exhibit stromal features, along with differentiation and cell replacement of adult neural progenitors, induction of oligodendrocyte fate decision, as well as immune modulation, neuro-protecting by paracrine effects, and increasing the re-myelinating activity (25,26).
Systemic lupus erythematosus (SLE)
MSCs appear to be a proper therapeutic approach, it has been demonstrated that MSCs derived from SLE patients have abnormalities in some phenotypes such as proliferation and differentiation. So allogeneic (rather than autologous) transplantation of MSCs from healthy donors, are capable to improve serological markers, stabilize renal functions and ameliorate the SLE complaint (22). MSCs exert regenerative, anti-inflammatory and specific trophic effects, not replacing abnormal tissues or differentiating into distinct cell lineages (27).
Rheumatoid arthritis (RA)
However, few clinical trials have been reported the role of MSCs to treat RA, MSCs could be an innovative, safe and effective therapeutic approach in controlling the refractory disease for regenerative potential and the anti-inflammatory property. On the other hand, there is a model for rheumatoid arthritis, as collagen-induced arthritis (CIA), which MSC therapy are not effective. Because MSCs stimulate cytokines associated with Th17, reversing the immunomodulatory properties of MSCs consequently worsen the clinical symptoms of CIA (22). Therefore, MSCs are only operational when administered at the beginning of disease. This issue reveals that MSCs immuno-regulatory properties will be abolished in presence of inflammatory microenvironment (6).
Type1 diabetes (T1D)
MSCs can generate populations of functional pancreatic β-cells for reloading supply of glucose-responsive insulin-producing cells. They have immunomodulation activity then opposing autoimmunity, ameliorate immune transplantation rejection (28). The MSCs implantation reduces the amounts of glucose by paracrine effect rather than direct regeneration of insulin-producing cells (29).
Cancers and MSC transplantation
MSCs are considered as a double-edged sword in cancer cell therapy. They exert supportive or suppressive effects on tumor growth. From one side, MSCs as a source of soluble factors have immune modulation activity, growth, and angiogenesis, then may possibly stimulate tumor expansion, metastasis, and anti-tumor immunity. On the other side, they inhibit survival signaling (apoptosis; via Wnt and Akt pathway), then may stop tumor growth. Nevertheless, MSCs with capability to home into tumor sites and to secrete cytokines can be recruited as a vehicle for the delivery of anti-tumor agents and therapeutic drugs (30). MSC-based anti-cancer therapy has also been recommended to be administered in form of engineered MSCs as novel anti-tumor carriers (31), by silencing and over expressing the genes, in favor or to the detriment of tumor, respectively. Thus far, other studies have been exerting MSCs against cancers (https://www.clinicaltrials.gov/).
Bone and cartilage diseases and MSC transplantation
Continual renewal and reparative properties of MSC mediated by paracrine mechanisms would enhance their regenerative effects and attenuate or feasibly correct genetic disorders of bone and cartilage tissues. MSCs differentiation into bone and cartilage are applicable in several methods such as systemic/local infusion or seeding MSCs on three-dimensional biodegradable Nano-scaffolds and the use of gene-modified MSCs, and hetero-MSCs application (32). The combination of MSCs, synthetic bone substitute, and platelet rich plasma would efficiently stimulate new bone formation (osteogenesis) and enriched in total body bone mineral content (33,34). Until now, 26 studies have been used MSCs for bone and cartilage diseases treatment (https://www.clinicaltrials.gov/).
Neuro-degenerative diseases and MSC transplantation
The capability of MSCs to trans-differentiate into neural cells would replace lost neurons and glia in neurodegenerative diseases. Furthermore, either intrinsic excretion or genetically over expressing of neurotrophic factors by MSCs promote regeneration of impaired tissue (35,36). MSCs’ neurotrophic factors and anti-inflammatory cytokines (GDNF, BDNF, NGF, IGF, TGF-β1, VEGF, IL-6, IL-10) activate neurogenesis, neuroprotection and immunomodulation in astrocytes, neurons and oligodendrocytes, additionally can inactivate cell death through apoptosis and diminish free radicals (37). As far as this, 29 studies have been recorded the usage of MSCs for neurodegenerative diseases treatment (https://www.clinicaltrials.gov/)
Kidney diseases and MSC transplantation
MSCs can mediate the protective and regenerative effects in the kidney repair through paracrine and endocrine mechanisms. They may possibly release trophic factors, which can promote kidney cells growth (mitogenesis), angiogenesis constrain cell death (apoptosis) and stimulate the resident stem cells of the kidney to repair itself (33). Thus far, 34 studies have been employed MSCs against kidney diseases (https://www.clinicaltrials.gov/). Highlights of therapeutic outcomes of MSC administrations in some clinical trials have been collected in Table 2.
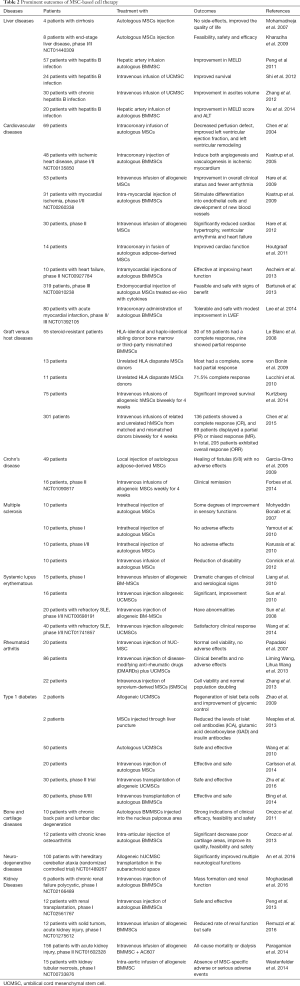
Full table
MSC therapy potential/theoretical risks
MSCs have been recruited in numerous approaches for immunomodulation and regenerative cell therapy. Hence, their biosafety features should be highly concerned to obliterate the functional or genetic alterations in clinical use. There are evidences that MSCs are capable of culturing long-term in vitro, without any changes in function, morphology, karyotype and phenotype (19).
Potential/theoretical risks of MSC therapy rely on many risk factors. These factors can be associated with; (I) the intrinsic cellular properties (the type or class of stem cells used); (II) extrinsic risk factors (the type and level of manipulation, precondition, culturing history, handling or storage of the cells); (III) the clinical characteristics (the type of surgical operation, immunosuppression, site and mode of administration). In the other words, potential risks of MSC therapy includes; tumorigenic potential, immune responses, pathogen transmission by MSCs (38). In addition to immune rejection and malignant transformation, MSCs administration have other adverse effects such as adipogenic differentiation and prothrombotic events (39). In other word, the potential risks of MSC administration can be categorized to; acute problems (as immune mediated reaction and embolic phenomenon), intermediate problems (as graft versus host disease and secondary infection) and long-term problems (as risk of malignancy).
MSCs probably are not spontaneously immunosuppressive, but require activation by inflammatory cytokines to exert their immunosuppressive effects. The immunosuppressive paracrine effects of MSCs can be likened to a double-edged sword. While suppression of cytokine production early in any disorders could be beneficial in reducing inflammation and organ damage, over-suppression of the protective cells such as B-cells and T-cells could be detrimental in later stages of the disease (2,3). The growth stimulation of formerly undetected tumors by MSCs has shown conflicting data of in vitro and in vivo as inhibition, enhancement, and no effect on tumor growth. Only a limited number of MSCs are present at the injury site after administration, but the destiny of the rest of the cells is still unclear. This issue increase the risk of ectopic grafting of MSCs (7).
For the risk of increase in secondary infections following MSC transplant due to the possible immunosuppressive effects, MSCs should be used with caution and adequate infection control. For the risk of the biosafety of growth medium components (such as FCS, FBS), the productions are going to use clinically should eliminate the use of FCS for the risk of transmission of pathogens, prions and zoonoses, instead platelet lysates can be used (7).
Manipulation of MSCs = MSCs modifications
Genetic modification of MSCs = genetically engineered MSCs
MSCs have also been genetically engineered/reprogramed to over express a desired gene to further improve their therapeutic efficacy. They can be utilized for the targeted delivery of therapeutic gene products as gene therapy. The genes possibly capable of manipulation could be receptors, growth factors and cytokines genes. Genetically engineered MSCs have been potentially utilized for treating a range of genetic or acquired diseases, as well as protein deficiencies, blood, cartilage, bone, cardiogenic disorders, neurological diseases, such as multiple sclerosis, Parkinson’s disease, or cerebrovascular disorders and feasibly even malignancies. Genetic modification of MSCs improves their therapeutic potential by augmenting various cellular manner such as endurance and survival of transplanted MSC, angiogenesis, differentiation, homing, and anti-inflammatory effects (40).
For instance, studies have investigated the role of introducing the pancreatic duodenal homeobox-1 (PDX-1) and VEGF genes into MSCs leading to differentiation into functional insulin-producing cells as cellular therapy for diabetes (41). Transduced MSCs with the β-glucuronidase (GUSB) gene improve genetic enzyme deficiency mucopolysaccharidosis type VII (MPSVII) (42).
Genetically engineered MSCs that express the IFN-βcan inhibit tumor cell growth. Moreover, transduced MSCs with tumor necrosis factor a (TNF-α) and interferon a (IFN-α) genes induce apoptosis, which is applicable for cancer therapy (40,43).
Genetically engineered MSCs over expressing the hHCN1 gene can modify the activity of cardiac pacemaker cells (44). Bcl-xL-MSCs also up-regulate expression of angiogenic cytokines (VEGF, IGF-1, and PDGF), which in turn stimulates angiogenesis (45). Overexpression of Bcl-2, heme-oxygenase-1 and Akt1 as anti-apoptotic genes, in MSCs improve the cell survival, helping heart tissue repair in myocardial infraction (40). Akt-overexpressing MSCs increasing secretion of frizzled-related protein 2 (SFRP2) and β-catenin, which activate anti-apoptotic gene transcription in is chemic cardiomyocytes, can lead to survival of myocardium.
MSCs modified with CXCR4 improves colonization rate of transplanted MSCs liver regeneration in acute liver failure (ALF). Moreover, MSCs-CXCR4, homing enhancement towards myocardium, are helpful to ameliorate the myocardial infarction. In myocardial infraction, angiopoietin-1-modified MSCs strengthen heart function and angiogenesis. In addition, calreticulin-modified MSCs intensify adhesion, migration and survival of the cell in MI (46).
Engineered MSCs to secrete numerous human cytokines, including IL-3, IL-7, SDF-1and SCF boost hematopoiesis in SCID (47). MSCs over expressing therapeutic cytokines IL-2, IL-7, IL-12, IL-18 and IL-23 can enhance the immune response to the tumor. IL-10 transduced MSCs are capable of reducing inflammatory response and improving survival post-transplant in GvHD (48).
MSCs transduced with bone morphogenetic proteins (BMPs), as potent inducers of osteogenic differentiation, have capability to repair many musculoskeletal defects. Osteoprotegerin (OPG)-transduced MSCs can diminish osteoclast activation and trabecular bone loss in bone myeloma. Furthermore, over expression of telomerase reverse transcriptase (TERT) in MSCS, accelerating reverse transcription (RT) and life span of the cells, help osteogenic proliferation and differentiation in osteoporosis treatment (45,49). Collagen type I protein modified MSCs effectively mend bones in osteogenesis imperfecta. As well, dystrophin-transfected MSCs contribute to myogenesis through cellular fusion and compensate the genetic defect of muscular dystrophy. Neurogenin1 (Ngn1) overexpression makes MSCs capable of inducing neuronal differentiation. MSCs over-express lipocalin 2 (Lcn2), reducing senescence, can restore renewal potential of MSCs (50).
Chemically engineered MSCs
Covalently conjugated cell adhesion molecules sialyl Lewis X (SLeX) on the MSC surface through a biotin-streptavidin bridge conveys leukocyte-like rolling characteristics without altering the cell phenotype and the multi-lineage differentiation potential but improve the targeting and homing efficiency of to specific tissue and induce a cell rolling response (51).
Preconditioning of MSCs
The mechanical injury and host inflammatory response cause the MSCs loss quickly following transplantation by apoptosis. Detached MSCs from the extracellular matrix, lack of nutritional materials and the activation of death receptors are factors triggering apoptosis cascades. Activation of apoptosis and release of pro-apoptotic factors, as well as cytochrome C (Cyt C), endonuclease G, caspase-3 and apoptosis-inducing factor (AIF) destroy the mitochondrial membrane led to cell death (52).
Improving the survival signals and resistance of MSCs against stress and insults in the pathological environment, cells had better to be preconditioned prior to implantation (11). For preconditioning, MSCs pretreat or expose to sub-lethal dose of various insults as well as hypoxia, toxins, reactive oxygen species (ROS), apoptotic cascade activation, inflammatory response, autophagy and many others. In addition to enhancing cell survival following transplantation, preconditioning considerably induces therapeutic benefits of MSCs by increasing the cell differentiation potential and its paracrine protective effect, enhancing migration and homing of transplanted cells to the lesion site, increasing regenerative and repair potentials, suppressing inflammatory and immune responses after transplantation (53).
Chemical preconditioning
Preconditioning by environmental stimuli: short-term exposure to brief oxidative, hypoxia or anoxia, hyperoxia, hydrogen peroxide, carbon monoxide, hydrogen sulfide and lithium chloride or the combination may possibly precondition the cells and induces an operative approach to improve resistance, survival and proliferation of MSCs to subsequent lethal ischemic injury (54). The significant down-regulated caspases 1, 3, 6, 7, and 9 up-regulated survival markers Nf-κB, Bcl-2 and Akt1, attenuate the apoptosis in preconditioned MSCs (55). Preconditioning with a combination of stress stimuli improves ischemic tolerance in various body tissues and stimulates production of some cytokines such as SDF-1, CXCR4 and VEGF and enhances migration of MSCs and recruitment of resistance MSCs (53,54,56).
Cytokine preconditioning, another approach to cell protection, can also be achieved using anti-apoptotic preconditioning strategies like interleukins (IL-1, IL-6) or Bcl-2 gene modification. Also, other cytokines as well as insulin like growth factor 1 (IGF-1), HGF, transforming growth factor (TGF), β-fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF), placental growth factor (PlGF), brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF), stromal cell-derived factor 1 (SDF1) or Angiopoietin-I treatment can be used in MSC preconditioning. Cytokine preconditioning of MSCs increases paracrine potentials and homing efficiency and improves the survival of the transplanted cells, enhances proliferation and differentiation, promotes angiogenesis and attenuates many pathophysiological changes (11,40,53,54).
Toxin preconditioning: MSCs preconditioning with low (appropriate) concentrations of lipopolysaccharide (LPS) has cyto-protective effect on apoptosis induced by subsequent high-dose LPS insults (57). In addition to enhancing survival of engrafted MSCs, LPS pretreatment induces expression of VEGF and subsequent neovascularization and stimulates PI3K/Akt pathway maximizing functional and biological features of MSCs. P-cresol uremic toxin also induces Akt-pathway-selective insulin resistance in MSCs (58).
Heat shock preconditioning: Hsp preconditioning maintains the stem cell potential, proliferation, and differentiation and promotes cell survival under oxidative stress and serum deprivation-induced apoptosis via the PI3K/Akt and ERK1/2 pathways and protects transplanted MSCs against aging (59,60).
Physical preconditioning
Low-intensity ultrasound preconditioning: MSCs exposed to Low-Intensity Ultrasound (LIUS) are prevented from apoptosis and enhanced viability and induced during chondrogenic differentiation (61).
Pharmacological preconditioning
MSC preconditioning with VPA (2-propylpentanoic acid) and desferrioxamine (DFX) increases their homing efficacy (62). Followings are some examples:
- Diazoxide preconditioning: pretreatment of MSCs with Diazoxide regulates NF-kB pathway and then improves MSCs’ survival by up-regulating pro-survival and anti-apoptotic genes and enhances regenerative potential by activating different angiogenic growth factors (63).
- Melatonin preconditioning: ex vivo pretreatment with melatonin improves post-transplantation survival, proangiogenic/mitogenic activity and the therapeutic effectiveness of MSCs. These cells stimulating the ERK signaling pathway diminishes brain infarction, and improves neuro-behaviors (64).
- Trimetazidine preconditioning: the survival rate Trimetazidine (TMZ) -pretreated MSCs are increased under hypoxic stimuli consequently promotes neovascularization and enhances recovery of myocardial function through up- regulating Bcl-2 expression (65).
- Sevoflurane preconditioning: sevoflurane, an inhaled anesthetic, used to MSCs preconditioning. It improves the therapeutic potential of MSCs, increasing survival and homing activity of MSCs against serum deprivation and hypoxia (66).
Future directions and perspectives
The recent progress in MSCs applications have made it an impressive appliance for regenerative medicine and upcoming cell therapy. Despite executing several clinical trials, fully MSCs mechanism of action is in their middling stages and there are still several unanswered questions. For instance, the most effective route of MSCs’ administration (local or systemic) survival and homing ability of MSCs after transplantation and the complete association between MSCs and the host immunity remains to be clarified. Even the mechanisms of the maintenance of MSCs proliferation and differentiation properties after transplantation are not obviously illuminated. However, several studies have recorded the successful transplantation, differentiation and homing of MSCs but it seems that their effect on diseases is much related to cytokines excretion rather than direct effect of the cells (19).
The future MSCs researches, updating the cell regeneration therapy, should address immunological or safety considerations in favor of the personalized approach, complete understanding of growth regulators in differentiation and trans-differentiation and site-specific homing, bio-banking strategies in large scale, finding more suitable markers to isolate the source-specific MSCs. In addition, there should be focus on the long-term safety of MSCs to minimize the risk of oncogenic transformation. Moreover, there should be paid more to find preconditioning strategies and genetic manipulation to improve survival of MSCs after transplantation. To address most features of MSCs therapeutic applications, including safety concerns, engraftment capability and rejection, further in vivo studies are still required (4). Hence, continuous efforts of researchers would make progress in the field then all kinds of diseases and damages would be repairable in the near future.
Conclusions
MSCs owing potential for multiple mechanisms of repair have many clinical implications for many different diseases and disorders. MSCs have multi-potentiality that may differentiate to replace damaged cells, paracrine effects that secrete bioactive factors for suppressing apoptosis, enhancing angiogenesis, performing immunomodulatory and anti-inflammatory effects and wound re-modeling. To date, systemic infusion of MSCs has been successfully used to ameliorate a variety of immune disorders, including GvHD, as well as neurodegenerative diseases, ameliorating hematopoietic stem cell (HSC) engraftment, SLE, tissue injury, diabetes, rheumatoid arthritis, autoimmune, lung, liver and heart diseases, inflammatory bowel disease, sepsis, and systemic sclerosis.
In conclusion, MSC therapy is at controversial breakthrough in treatment and amelioration of the devastating and until now incurable disease. So far, many preclinical and clinical studies using MSCs have been accomplished, but before therapeutic using them on vast clinical scale, there are some issues should have concerned. First, the long-term safety of using MSCs must be determined. Next, quality control and clinical grade production are necessary before in vivo application of MSCs, according to supplementary tests, such as cell viability, endotoxin and oncogenic assays. Then the optimum dose and precise administration time should be concerned depend on the harshness of each disease. Lastly, comprehensive understanding the fundamental mechanisms of action, manipulations and preconditioning to produce more safe and effective MSCs for cell therapy.
New guideline is suggested for MSC therapy in hopes of improving their therapeutic efficacy. A combination strategy could be interesting and promoting the perception of MSCs potentials, functions and clinical perspectives. These strategies are manipulations and preconditioning, and inspecting potential/unexpected risks. The potential risks would probably include undesirable immune responses, tumor formation and the transmission of incidental agents.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Wei X, Yang X, Han ZP, et al. Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacologica Sinica 2013;34:747-54. [Crossref] [PubMed]
- Wannemuehler TJ, Manukyan MC, Brewster BD, et al. Advances in mesenchymal stem cell research in sepsis. J Surg Res 2012;173:113-26. [Crossref] [PubMed]
- Chen C. From mesenchymal stem cell therapy to discovery of drug therapy for systemic sclerosis. University of Southern California, ProQuest Dissertations Publishing, 2014. 3628136.
- Hirvonen T. Glycan Binding Proteins in Therapeutic Mesenchymal Stem Cell Research. 2014. Available online: https://helda.helsinki.fi/bitstream/handle/10138/135978/glycanbi.pdf?sequence=1
- Javaregowda PK, Yoon JW, Jang G. Roles of Mesenchymal Stem Cells (MSCs) in bacterial diseases. J Biomed Res 2013;14:184-94. [Crossref]
- Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells-current trends and future prospective. Biosci Rep 2015;35. [Crossref] [PubMed]
- Kusadasi N, Groeneveld AJ. A Perspective on Mesenchymal Stromal Cell Transplantation in the Treatment of Sepsis. Shock 2013;40:352-7. [Crossref] [PubMed]
- Ripoll CB. Adult stem cell therapy in the twitcher mouse model of Krabbe's disease utilizing mesenchymal lineage stem cells: Tulane University; 2010. Available online: https://digitallibrary.tulane.edu/islandora/object/tulane%3A26064
- Halabian R, Roudkenar MH, Jahanian-Najafabadi A, et al. Co-culture of bone marrow-derived mesenchymal stem cells overexpressing lipocalin 2 with HK-2 and HEK293 cells protects the kidney cells against cisplatin-induced injury. Cell Biol Int 2015;39:152-63. [Crossref] [PubMed]
- Halabian R, Tehrani HA, Jahanian-Najafabadi A, et al. Lipocalin-2-mediated upregulation of various antioxidants and growth factors protects bone marrow-derived mesenchymal stem cells against unfavorable microenvironments. Cell Stress Chaperones 2013;18:785-800. [Crossref] [PubMed]
- Augustin MM. Preconditioning Methods in Cell Therapy of the Heart. Conference Proceedings, 2012.
- Van Poll D, Parekkadan B, Rinkes IB, et al. Mesenchymal stem cell therapy for protection and repair of injured vital organs. Cell Mol Bioeng 2008;1:42-50. [Crossref]
- Hamedi-Asl P, Halabian R, Bahmani P, et al. Adenovirus-mediated expression of the HO-1 protein within MSCs decreased cytotoxicity and inhibited apoptosis induced by oxidative stresses. Cell Stress Chaperones 2012;17:181-90. [Crossref] [PubMed]
- Mohammadzadeh M, Halabian R, Gharehbaghian A, et al. Nrf-2 overexpression in mesenchymal stem cells reduces oxidative stress-induced apoptosis and cytotoxicity. Cell Stress Chaperones 2012;17:553-65. [Crossref] [PubMed]
- Kiani AA, Kazemi A, Halabian R, et al. HIF-1α confers resistance to induced stress in bone marrow-derived mesenchymal stem cells. Arch Med Res 2013;44:185-93. [Crossref] [PubMed]
- Kim N, Cho SG. Clinical applications of mesenchymal stem cells. Korean J Intern Med 2013;28:387-402. [Crossref] [PubMed]
- Kean TJ, Lin P, Caplan AI, et al. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int 2013;2013:732742.
- Del Papa N, Di Luca G, Sambataro D, et al. Regional Implantation of Autologous Adipose Tissue-Derived Cells Induces a Prompt Healing of Long-Lasting Indolent Digital Ulcers in Patients with Systemic Sclerosis. Cell Transplant 2015;24:2297-305. [Crossref] [PubMed]
- Volarevic V, Nurkovic J, Arsenijevic N, et al. Concise review: Therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells 2014;32:2818-23. [Crossref] [PubMed]
- Trivedi P, Tray N, Nguyen T, et al. Mesenchymal stem cell therapy for treatment of cardiovascular disease: helping people sooner or later. Stem Cells Dev 2010;19:1109-20. [Crossref] [PubMed]
- Bernstein HS, Srivastava D. Stem cell therapy for cardiac disease. Pediatr Res 2012;71:491-9. [Crossref] [PubMed]
- Kim N, Cho S. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells 2015;8:54-68. [Crossref] [PubMed]
- Tolar J, Villeneuve P, Keating A. Mesenchymal stromal cells for graft-versus-host disease. Hum Gene Ther 2011;22:257-62. [Crossref] [PubMed]
- Amorin B, Alegretti AP, Valim V, et al. Mesenchymal stem cell therapy and acute graft-versus-host disease: a review. Human cell 2014;27:137-50. [Crossref] [PubMed]
- Connick P, Kolappan M, Crawley C, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol 2012;11:150-6. [Crossref] [PubMed]
- Rivera FJ, Aigner L. Adult mesenchymal stem cell therapy for myelin repair in multiple sclerosis. Biol Res 2012;45:257-68. [Crossref] [PubMed]
- Carrion FA, Figueroa FE. Mesenchymal stem cells for the treatment of systemic lupus erythematosus: is the cure for connective tissue diseases within connective tissue? Stem Cell Res Ther 2011;2:23. [Crossref] [PubMed]
- Chhabra P, Brayman KL. Stem Cell Therapy to Cure Type 1 Diabetes: From Hype to Hope. Stem Cells Transl Med 2013;2:328-36. [Crossref] [PubMed]
- Katuchova J, Harvanova D, Spakova T, et al. Mesenchymal stem cells in the treatment of type 1 diabetes mellitus. Endocr Pathol 2015;26:95-103. [Crossref] [PubMed]
- Hong IS, Lee HY, Kang KS. Mesenchymal stem cells and cancer: Friends or enemies? Mutat Res 2014;768:98-106. [Crossref] [PubMed]
- Yagi H, Kitagawa Y. The Role of Mesenchymal Stem Cell in Cancer Development. Front Genet 2013;4:261. [Crossref] [PubMed]
- Mobasheri A, Kalamegam G, Musumeci G, et al. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas 2014;78:188-98. [Crossref] [PubMed]
- Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int 2013;2013:496218.
- Qin Y, Guan J, Zhang C. Mesenchymal stem cells: mechanisms and role in bone regeneration. Postgrad Med J 2014;90:643-7. [Crossref] [PubMed]
- Tanna T, Sachan V. Mesenchymal stem cells: potential in treatment of neurodegenerative diseases. Curr Stem Cell Res Ther 2014;9:513-21. [Crossref] [PubMed]
- Blundell R, Shah M. Neurodegenerative Diseases and Stem Cell Transplantation. J Stem Cell Res Ther (Edmond) 2015;5:277.
- Colpo GD, Ascoli BM, Wollenhaupt-Aguiar B, et al. Mesenchymal stem cells for the treatment of neurodegenerative and psychiatric disorders. An Acad Bras Cienc 2015;87:1435-49. [Crossref] [PubMed]
- Herberts CA, Kwa MSG, Hermsen HPH. Risk factors in the development of stem cell therapy. J Transl Med 2011;9:29. [Crossref] [PubMed]
- Eirin A, Lerman LO. Mesenchymal stem cell treatment for chronic renal failure. Stem Cell Res Ther (Walnut) 2014;5:83. [Crossref] [PubMed]
- Hodgkinson CP, Gomez JA, Mirotsou M, et al. Genetic Engineering of Mesenchymal Stem Cells and Its Application in Human Disease Therapy. Hum Gene Ther 2010;21:1513-26. [Crossref] [PubMed]
- Hashemian SJ, Kouhnavard M, Nasli-Esfahani E. Mesenchymal Stem Cells: Rising Concerns over Their Application in Treatment of Type One Diabetes Mellitus. J Diabetes Res 2015;2015:675103.
- Meyerrose TE, Roberts M, Ohlemiller KK, et al. Lentiviral-Transduced Human Mesenchymal Stem Cells Persistently Express Therapeutic Levels of Enzyme in a Xenotransplantation Model of Human Disease. Stem cells 2008;26:1713-22. [Crossref] [PubMed]
- Zhao W, Sarkar D, Ankrum J, et al. Therapeutic applications of mesenchymal stem/multipotent stromal cells. In: Appasani K., Appasani R. (eds). Stem Cells & Regenerative Medicine. Humana Press, Totowa, 2011. p. 195-218.
- Zhou YF, Yang XJ, Li HX, et al. Genetically-engineered mesenchymal stem cells transfected with human HCN1 gene to create cardiac pacemaker cells. J Int Med Res 2013;41:1570-6. [Crossref] [PubMed]
- Xue X, Liu Y, Zhang J, et al. Bcl-xL Genetic Modification Enhanced the Therapeutic Efficacy of Mesenchymal Stem Cell Transplantation in the Treatment of Heart Infarction. Stem Cells Int 2015;2015:176409.
- Ma HC, Shi XL, Ren HZ, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 to acute failing liver improves liver regeneration. World J Gastroenterol 2014;20:14884-94. [Crossref] [PubMed]
- Chen T, Zhang P, Fan W, et al. Co-transplantation with mesenchymal stem cells expressing a SDF-1/HOXB4 fusion protein markedly improves hematopoietic stem cell engraftment and hematogenesis in irradiated mice. Am J Transl Res 2014;6:691. [PubMed]
- Namba H, Kawaji H, Yamasaki T. Use of genetically engineered stem cells for glioma therapy. Oncol Lett 2016;11:9-15. [Crossref] [PubMed]
- Amini AR, Laurencin CT, Nukavarapu SP. Bone Tissue Engineering: Recent Advances and Challenges. Crit Rev Biomed Eng 2012;40:363-408. [Crossref] [PubMed]
- Bahmani B, Roudkenar MH, Halabian R, et al. Lipocalin 2 decreases senescence of bone marrow-derived mesenchymal stem cells under sub-lethal doses of oxidative stress. Cell Stress Chaperones 2014;19:685-93. [Crossref] [PubMed]
- Sarkar D, Zhao W, Gupta A, et al. Cell surface engineering of mesenchymal stem cells. Methods Mol Biol 2011;698:505-23. [Crossref] [PubMed]
- Lu HH, Li YF, Sheng ZQ, et al. Preconditioning of stem cells for the treatment of myocardial infarction. Chin Med J (Engl) 2012;125:378-84. [PubMed]
- Yu SP, Wei Z, Wei L. Preconditioning strategy in stem cell transplantation therapy. Transl Stroke Res 2013;4:76-88. [Crossref] [PubMed]
- Khan M, Ali F, Mohsin S, et al. Preconditioning diabetic mesenchymal stem cells with myogenic medium increases their ability to repair diabetic heart. Stem Cell Res Ther 2013;4:58. [Crossref] [PubMed]
- Saini U, Gumina RJ, Wolfe B, et al. Preconditioning mesenchymal stem cells with caspase inhibition and hyperoxia prior to hypoxia exposure increases cell proliferation. J Cell Biochem 2013;114:2612-23. [Crossref] [PubMed]
- Haider HK, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol 2008;45:554-66. [Crossref] [PubMed]
- Hou YS, Liu LY, Chai JK, et al. Lipopolysaccharide pretreatment inhibits LPS-induced human umbilical cord mesenchymal stem cell apoptosis via upregulating the expression of cellular FLICE-inhibitory protein. Mol Med Rep 2015;12:2521-8. [Crossref] [PubMed]
- Noh H, Yu MR, Kim HJ, et al. Uremic Toxin p-Cresol Induces Akt-Pathway-Selective Insulin Resistance in Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells 2014;32:2443-53. [Crossref] [PubMed]
- Gao F, Hu XY, Xie XJ, et al. Heat shock protein 90 protects rat mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis via the PI3K/Akt and ERK1/2 pathways. J Zhejiang Univ Sci B 2010;11:608-17. [Crossref] [PubMed]
- Fan GC. Role of Heat Shock Proteins in Stem Cell Behavior. Prog Mol Biol Transl Sci 2012;111:305-22. [Crossref] [PubMed]
- Ling L, Feng X, Wei T, et al. Effects of low-intensity pulsed ultrasound (LIPUS)-pretreated human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation on primary ovarian insufficiency in rats. Stem Cell Res Ther (Walnut) 2017;8:283. [Crossref] [PubMed]
- Bidkhori HR, Ahmadiankia N, Moghaddam Matin M, et al. Chemically primed bone-marrow derived mesenchymal stem cells show enhanced expression of chemokine receptors contributed to their migration capability. Iran J Basic Med Sci 2016;19:14-9. [PubMed]
- Afzal MR, Haider HK, Idris NM, et al. Preconditioning Promotes Survival and Angiomyogenic Potential of Mesenchymal Stem Cells in the Infarcted Heart via NF-κ B Signaling. Antioxid Redox Signal 2010;12:693-702. [Crossref] [PubMed]
- Tang Y, Cai B, Yuan F, et al. Melatonin pretreatment improves the survival and function of transplanted mesenchymal stem cells after focal cerebral ischemia. Cell Transplant 2014;23:1279-91. [Crossref] [PubMed]
- Hu X, Yang J, Wang Y, et al. Mesenchymal stem cells preconditioned with trimetazidine promote neovascularization of hearts under hypoxia/reoxygenation injury. Int J Clin Exp Med 2015;8:16991. [PubMed]
- Sun X, Fang B, Zhao X, et al. Preconditioning of mesenchymal stem cells by sevoflurane to improve their therapeutic potential. PloS One 2014;9:e90667. [Crossref] [PubMed]
Cite this article as: Saeedi P, Halabian R, Imani Fooladi AA. A revealing review of mesenchymal stem cells therapy, clinical perspectives and Modification strategies. Stem Cell Investig 2019;6:34.


