
Periostin expression and characters of human adipose tissue-derived mesenchymal stromal cells were aberrantly affected by in vitro cultivation
Introduction
Mesenchymal stromal cells (MSCs) population, isolated from bone marrow (BM), is still considered as the gold standard for MSC applications. However, the several limitations of BM, harvesting BM-derived MSCs (BM-MSCs) by aspiration technique is a painful and tedious method. Surprisingly, in other human tissues like adipose tissue, identification and isolation of MSCs with BM-MSCs-like characters has been found (1). Adipose tissue-derived MSCs (AD-MSCs) are “an attractive alternative” to BM-MSCs, these cells have gained more attention over the last years since they can be safely and easily harvested with minimal morbidity in large quantities. The amount of stem cells derived from adipose tissue is 100 folds more than that derived from BM in addition to their multiple differentiation capacity and biological characteristics (2). On the basis of proliferation rate, AD-MSCs were faster than BM-MSCs. Moreover, MSCs purified from different tissues have different inflammatory, angiogenic, and matrix remodeling potential characters. However, the mechanisms controlling the aging of stem cell still remain a mystery. Thus, MSCs from older patients may be actually harm them.
It was reported that AD-MSCs are heterogeneous cells and exhibit variations in population doubling time (PDT), morphology and proliferative capacity. Furthermore, cell heterogeneity might be resulting from aneuploidy (aberrant content of DNA) which is a form of genetic instability, these changes might be considered a hallmark of tumorigenic potential, so genomic instability might be responsible for tumor cells characteristics (3), thus the most important issue for clinical use of MSCs is the tumorigenesis potential (4). Thus, we investigated if cellular phenotypes instability may lead to the deterioration of tissues structure and their function, then may give rise to malignant changes in these cells. Human BM-MSC studies reported highly paradoxical results. It has been shown that human BM-MSCs might express tumor transformation in vivo as well as chromosomal aberrations in vitro (5). Nevertheless, other authors have reported no malignant transformation in vivo and normal karyotype during human BM-MSC in vitro culture (6). Others observed irreversible cell growth arrest due to cellular senescence (7). Although some reports showed the alarming finding of human MSCs malignant transformation (8), there is still a great argumentation concerning genetic stability of human MSCs and their influence on clinical safety.
Periostin, a component of the extracellular matrix (ECM), has a role in promoting migration, proliferation, cell survival and adhesion. Although periostin over-expression is clearly observed in some cancers, it is not a general trait of tumors. In addition, periostin has been associated with lymph node metastasis, tumor size, lymphatic invasion, and disease stage in non-small cell lung cancer patients (9). To date, there is no available quantitative comparison of periostin expression in a large panel of tumor and normal cells. Furthermore, involvement of human adipose MSC-secreted periostin in tumorigenesis has not been explored.
To date, no standardized protocol or dose of MSCs in stem cell transplantation, thus a great debate is present about how to choose the best standard protocols for their clinic therapeutic use. Nevertheless, several studies confirmed the beneficial effects of higher number of transplanted cells versus low number in different clinical applications, thus requiring prolonged cultivation and extensive cell expansion (10).
This study investigated if human AD-MSCs are developing, during in vitro long-term cultivation, in an unwanted or aberrant way. To achieve this, we monitored AD-MSCs during their in vitro culture till the tenth passage investigating their proliferation kinetics, immunophenotype, surface markers expression as determined by The International Society for Cellular Therapy (ISCT) (11), and genetic stability and DNA index (DI) as proposed by The European Medicine Agency (EMA) (12). Also, we investigated if there is any abnormal change of periostin gene expression between different passages, especially in late passages, during cultivation. Our results highlight the AD-MSCs proliferation capacity and their genomic safety. Also, we reveal a novel marker for potential senescence of AD-MSCs during in vitro prolonged cultivation.
Methods
A total number of 20 donors (male, age: 35–50 years) have been undergone abdominal liposuction. By liposuction aspirates, human subcutaneous lipoaspirate samples were obtained. Tissues were harvested under loco-regional anesthesia associated with light sedation by an experienced surgeon. Written and verbal informed consent was obtained from all donors, who were free from HBV and HIV and non-smokers, prior to enrollment in the study. Abdomen infiltration was performed in a 1:1 ratio (1 mL of aspirated tissue per 1 mL of solution composed of epinephrine 1:1,000,000 in Ringers solution). Then, adipose tissue was aspirated, using a 50 mL syringe attached to a 3-mm diameter blunt cannula, from the lower abdomen.
Isolation of MSCs from adipose tissue
The subcutaneous adipose tissues were isolated as previously described (13). The work was done during a period from January, 2017 to March, 2018. Briefly, after liposuction to eliminate contaminants like blood cells, it was vigorously washed with Dulbecco phosphate-buffered saline (DPBS; Invitrogen) containing 1% of penicillin/streptomycin (Invitrogen). Then, with 0.1% collagenase type I (GIBCO, cat. no. 17100), the samples were treated for 1 h in a shaking water bath at a speed of (120 rpm) to digest the ECM. Cell pellets were obtained by centrifugation at 1,500 rpm for 5 minutes. To remove debris, pellets were re-suspended and filtered using 100 µm mesh filter (BD Pharmingen, San Diego CA, USA). After discarding the suspending layer with the lipid droplets, pellets were re-suspended and washed twice. Using red blood lysis buffer (Sigma Aldrich), contaminating erythrocytes were lysed and the residual cells (106 cells /flask) were transferred to T75 cm2 culture flasks (BD Pharmingen) with culture medium containing 1% penicillin/streptomycin (Invitrogen), KO-DMEM 0.5%, 10% FBS (Hyclone) and 1% 1× Glutamax (Invitrogen) with humidified atmosphere of 5% CO2 at 37 °C. Non-adherent cells were removed, after 48 h, and medium was changed twice a week until 80–90% confluence. The mesenchymal population was identified on the basis of its plastic adhere to the bottom of the flask and morphology.
Cell cultivation
Non-adherent cells were removed, after 48 h, and medium was changed twice a week until 80–90% confluence. Using trypsin-EDTA solution (0.25%, Sigma Aldrich, USA), cell passaging was performed. Cellular viability and the number of recovered cells evaluated with the use of hemocytometer were quantified by the Trypan Blue exclusion test. Approximately 3×105 cells were used to inoculate 75 cm2 culture flasks and incubated at 37 °C in a humidified 5% CO2 incubator. Cell cultivation was performed up to the tenth passage (14).
Analysis of cell proliferation
To determine the proliferation rate, a total of 1,000 cells/cm2 were seeded in T-25 flasks (BD Pharmingen). Each time point and passage had three replicates. Cells were trypsinized after reaching 90% confluence. Next, by trypan blue dye exclusion, the cell numbers were calculated in each passage and assessed for viability. Cells were re-plated for subsequent passages and a total of ten passages were investigated in this study. Growth kinetics of passages was estimated by:
PDT
Using the formula: population doubling time (PDT) = tlg2/(lgNH − lgNI), PDT was calculated, where t represents the duration of the culture (in hours), NH the cell harvest number and NI the inoculum cell number (14).
Colony forming unit (CFU) assay
It measures the capability of MSCs of different passages to perform colonies. CFU was estimated by culturing 100 cells in a 35-mm dish (BD Bioscience), for variable periods (according to each passage), in 5% CO2 humidified incubator at 37 °C. Cells were washed twice using DPBS-Mg2+, -Ca2+ (Invitrogen) and fixed for 20 minutes using 100% methanol (Mallindkrodt, Hazelwood, USA) at room temperature, followed by staining with 3% crystal violet (Sigma Aldrich). Using tap water, cells were washed until the dishes became colourless. The dishes were air-dried for several minutes after inversion downwards on a clean cloth. CFU was estimated as following; CFU = (total number of stained colonies >2 mm/initial number of cells) ×100% (14).
Cell proliferation assay using MTT
To correlate between the expression of periostin and cell proliferation, a colorimetric 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) assay was used. The MTT assay, that replaced the radioactive tritiated thymidine incorporation assay, was the first widely accepted method to measure cell proliferation. After 4 days of culture, MTT solution was added and the cells were incubated in 5% CO2 humidified incubator at 37 °C for 4 h. Then the formazan solubilization solution was added and the absorbance was detected at 450 nm.
Flow cytometric characterization of in vitro cultured AD-MSCs
The following monoclonal antibodies (mouse antihuman), purchased from BD Biosciences (San Jose, CA, USA), were used for flow cytometric characterization of MSCs: CD90, CD34, CD73, CD45, and CD105 conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE). FACS Canto II flow cytometer (BD Biosciences) was used for sample acquisition and FACS Diva 6.1 software was used for the analysis.
Cells were trypsinized, after reaching 90% confluence, and re-suspended in DPBS (Invitrogen). A 1×106 cell suspension was incubated in dark with labeled antibodies at 37 °C for 1 h. Cells were characterized using the following conjugated monoclonal antibody combinations: CD34/CD45/CD90/CD73/CD105. Negative controls of FITC- or PE-conjugated corresponding immunoglobulins were used. Then samples were washed and acquired immediately after staining. A total 100,000 events were acquired, at low speed, after the cytometer was compensated and calibrated.
Flow cytometry-mediated analysis of DNA aneuploidy in in vitro cultured MSCs
Flow cytometry was performed to calculate the cell percentages in each cell cycle phase and to measure the DI and the aneuploidy/euploid DNA content ratio. The cytometric estimation of the degree of DNA content abnormality is commonly represented by DI which is the ratio of Go/G1 peak channel of the aneuploidy cells with normal DNA diploid cells. A normal diploid DNA content is represented by DI of 1.0, whereas deviation in cellular DNA content values other than 1.0 indicates DNA aneuploidy or polyploidy. When at least two separate G0/G1 peaks are detected, the occurrence of DNA aneuploidy was reported.
For DNA ploidy analysis we used BD cycle test TM plus DNA kit (BD; San Jose, CA 95131, USA). About 10,000 cells in suspension were centrifuged at 400 g at room temperature (20–25 °C) for 5 minutes. After discarding the supernatant, the sediment cell pellets were suspended and incubated for 10 minutes in 250 µL of trypsin buffer (Solution A) at room temperature. After that 200 µL of trypsin inhibitor was added with RNase buffer (Solution B) without aspiration of solution A and incubate them at room temperature for 10 minutes. Then, to each tube, 200 µL of cold propidium iodide (PI) was added and gently mixed. Finally, tubes were incubated in dark at (2–8 °C). Samples are now ready to be analyzed by flow cytometry. The identification and quantification of DNA aneuploidy/euploidy states and the cell cycle phase calculation were accomplished with the aid of the ModFit LT software (15).
RT-PCR for periostin gene expression
Periostin gene expression was analyzed using the Human TaqMan assay (Applied Biosystems, USA) which contains a known set of proven gene expression markers. Total RNA was stocked at −80 °C after extraction using TRIzol (Invitrogen) according to the manufacturer’s instructions. After treatment with DNase I (Ambion) and RNase-free ribonuclease inhibitor, cDNA was synthetized from RNA (500 ng) according to the manufacturer’ instructions by using SuperScript II Reverse Transcriptase (Invitrogen). cDNAs were loaded on to the StepOnePlus (Applied Biosystems). Periostin gene expression was normalized to GAPDH expression. Transcriptional analysis for cells of different passages was performed. For data analysis, SPSS was used to estimate the levels of target cells (different passages) gene expression relative to the calibrator (GAPDH) expression level with comparative CT method (ΔΔCT). A 35 cutoff cycle threshold (Ct) value was set, for estimation of the fold change, so a Ct value above 35 was deemed to be undetected (16). The primers and fluorogenic probes (Applied Biosystems) used are as following:
- GAPDH: (F) 5'-CTGCCCCTTCTGCTGATGC-3'; (R) 5'-GACAACTTCGGCATCGTGGA-3';
- Periostin: (F) 5'-GCTGCTGTTCCTGTGTGACG-3'; (R) 5'-TGACCCCGCAAATGCCAACAGTT-3';
- Probe: GCTGTGAGCTAGGACCTTGTCATAG.
Hs 01566750 Applied Biosystems TaqMan Gene Expression assays are used for quantitative real-time PCR analysis of gene expression containing a pair of unlabeled PCR primers and a TaqMan probe with a dye label (FAM) on the 5' end and a minor groove binder (MGB) and non-fluorescent quencher (NFQ) on the 3' end.
Statistical analysis
Results were obtained from at least three independent experiments. Data was expressed as mean ± standard deviation. Using GraphPad Prism 5 statistical software (GraphPad, La Jolla, CA) and Excel software (Microsoft, Redwood, WA, USA), Differences were analyzed by Student’s t-test after one-way analysis of variance (ANOVA). Differences were considered significant when the probability values (P) were less than 0.01.
Results
Morphology of AD-MSCs during in vitro culture
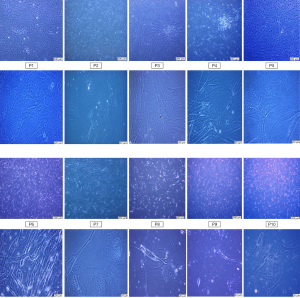
AD-MSCs were microscopically observed at every passage. Adherent flat fibroblast-like cells or long spindle-shaped cells were checked 24–48 h after isolation and kept to passages 6 (P6). AD-MSCs showed ability to form multilayer after confluence. Later on the portion of enlarged cells with changed morphology was gradually increased, the volume of the MSCs increased gradually with increasing passage number and the cells became flattened, making it is difficult for them to form a single monolayer cell growth tended to become slower which became obvious at late passages 7–10 (P7–P10), as shown in Figure 1.
Proliferation kinetics of AD-MSCs
PDT
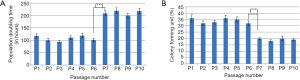
Significant increase in PDT was observed in passage 7 compared to passage 6 (P<0.001) indicating more rapid rate of proliferation of early passages until P6. Based on the results, shown in Figure 2A, early passages, P1, P2, P3, P4, P5, and P6, tended to double their population in 117.0±8.9, 100.0±7.1, 93.0±4.4, 110.0±8.0, 119.0±8.9, and 100.0±7.1 h, respectively, while those from late passages, P7, P8, P9, and P10, exhibited a doubling time of 208.0±9.8, 219.0±12.0, 200.0±9.8, and 218.0±11.1 h, respectively.
Determination of cell-colony formation
CFU assay is a suitable tool for evaluating the colonogenic capacity and proliferation of the passages of MSC cells expanded in culture. Significant reduction in capability of clonality was observed in passage 7 compared to passage 6 (P<0.001). The percentages of colony formation for cells of passages 6 and 7 are 32%±0.019% and 20%±0.001%, respectively, as shown in Figure 2B.
Cell viability
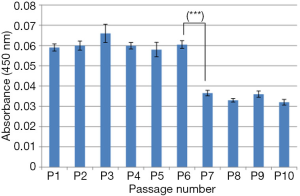
To evaluate the viability of AD-MSCs cultured from different passages, MTT assay was used. The representative absorbance of the MSCs is shown in Figure 3. P7 showed significantly low cell viability (P<0.001) when compared to P6. This result indicates that cell viability decreased with increasing age of cells in culture.
Immunophenotypic characterization
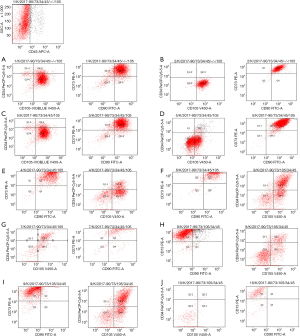
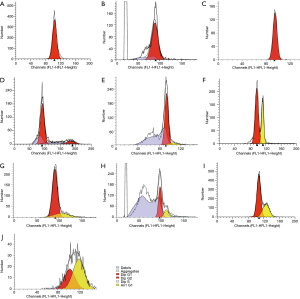
Flow cytometric analysis revealed that MSCs were identified based on the following phenotype: CD105+, CD73+, CD90+, CD34−, CD45−. In the following representative dot plots of in vitro cultured ten passages of adipose tissue, by gating on the CD45 negative population as shown in Figure 4, we found that MSCs markers (CD90, CD73, CD105) are strongly expressed in passage 1 up to passage 4 (Figure 4A,B,C,D). These results confirmed that cells from all AD-MSCs passages express MSC surface markers, as defined by the ISCT. Partial loss and weak expression of CD105 began in passage 5 until it completely lost in passage 10. Also, a newly differentiated population, that expressed both CD105 and CD34 markers, appeared in passage 5 (Figure 4E). Furthermore a partial loss of CD73 and CD90 expression began in passage 8 till complete loss of CD90 in passage 10 (Figure 4H,I,J). Table 1 showed the immunophenotypic characterization of MSCs in the ten passages.
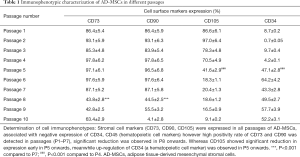
Full table
Cell cycle and DNA analysis for the different passages of AD-MSCs
DNA histograms obtained using flow cytometry, in Figure 5, showed the distribution of cycling MSCs in the various phases of cell cycle. The DNA histograms of PI-tagged MSCs emitting bright red fluorescence provided data for the cell cycle: G0/G1 (2N DNA content), S (between 2N and 4N), and G2/M (4N). We found that normal diploid DNA content in passage 1 up to passage 4. DNA aneuploidy cells appeared but with low percentage in passage 5. In passages 1, 2, 3 and 4, 100% of cells show normal diploid DNA (DI =1) which is represented by single G0/G1 peak. S-phase (proliferation fraction) is 0%. In passages 5, 6, 7, 8, 9 and 10, 91.20%, 64.85%, 79.24%, 74.76%, 64.90% and 54.39% of cells show normal diploid DNA content, respectively, with DI about 1.16. Distribution of cell cycle phases in in-vitro cultured AD-MSCs are shown in Table 2.
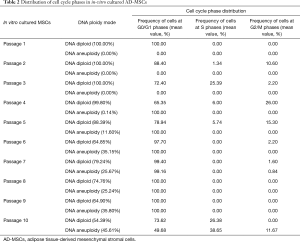
Full table
Periostin gene expression
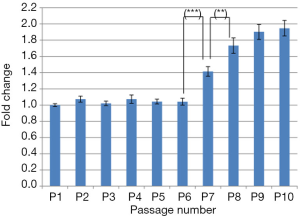
For further evaluation of the AD-MSCs, the expression of periostin gene was measured using commercial qPCR arrays for all passages, normalized to the internal control GAPDH, as shown in Figure 6. Significant up-regulation was detected in P7 (P<0.001) when compared to P6 and progressively increased onwards. Also, significant up-regulation was observed in P8 (P<0.01) when compared to P7 whereas non-significant increase was observed in P9 and P10 when compared to P8 and P9, respectively.
Discussion
AD-MSCs are widely-used adult stem cells that are considered one of the ideal resources for cell therapy. However, there are many unresolved issues concerning the clinical application of AD-MSCs, including the fact that cell numbers and genetic status were influenced by age (17). Therefore, researchers are trying to investigate the most suitable passages for cell therapy.
Although MSCs could be successfully propagated to advanced passages, the resulting cells showed noticeable differences in cultivation rates and proliferation kinetics. Previous studies showed that AD-MSCs accounted for 2% of karyocytes (18). To date, there is no specific molecular marker available for accurate determination of the cellular aging degree in MSCs (19,20). It has been reported that marrow-derived MSCs express periostin which is an ECM associated protein. Its expression is regulated and associated with several tissues’ ontogeny, like dental ligament, heart, and bone (21). It is also highly expressed in physiological and pathological conditions. It acts as an osteoblast specific factor during remodeling tissues, and expressed in bone tissues as disulfide liked 90 kDa protein. Furthermore, it works through promotion of cell migration and angiogenesis after binding to some integrins (22). Periostin regulates differentiation and cell adhesion of osteogenic cells and acts as an adhesion protein (23). It is up-regulated in many cancers and plays various important roles. Moreover, in tumor cells its expression is associated with a poor prognosis (24).
Real-time PCR was used to estimate periostin gene expression. We observed significant up-regulation in its expression in P7 and P8. These results further confirming periostin role as a powerful indicator to assess MSCs cellular aging and to estimate efficiency and safety of long-term cultivation. Moreover, BM-MSCs might exert their role through periostin action in gaining multipotent stem cell-like characters in breast cancer cells (25). Thus, molecular analysis of periostin gene expression might provide a powerful tool, during long-term expansion, to track cellular senescence of MSCs (26). It has been reported that BM-MSCs significantly promoted carcinogenesis by enhancing cell migration, epithelial-mesenchymal transformation (EMT), proliferation, inhibition of apoptosis, and altering expression of proteins regulating cell cycle (27). Meanwhile the tumor-promoting molecular mechanisms of BM-MSCs on some cancers like head and neck cancer are not clear.
By measuring DI, we have reported the quantification of the DNA diploidy and aneuploidy distribution and DNA heterogeneity in flow cytometrically analyzed passages of AD-MSCs. Furthermore, the cell percentages in different phases and the measurement of the DI were estimated. Interestingly, the total rates of DNA-diploid S-phase cell fractions were the highest in passage 4, suggesting that enhanced DNA replicative activity might occur in this passage. This finding can be correlated with the increased mitotic ability of the cells and so proliferation. However, this percentage significantly reduced in passage 5, also highly significant reduction was observed in passage 6 and progressive reduction was observed till complete disappearance of S-phase cells in passage 10. Additionally, a higher incidence of DNA-aneuploid cells was observed in our study, especially in late passages. In passage 1, 100% of cells showed normal diploid DNA (DI =1) which is represented by single G0/G1 peak corresponding to the same channel of the biological control. This percentage began to decrease from passage 4 where 99.89% of cells showed normal diploid DNA content. Significant reduction in cells with normal diploid DNA content (88.39%) was observed in passage 5 in comparison to 11.6% of cells which have abnormal DNA content (DNA aneuploidy). Marked reduction in cells of normal diploid DNA content was observed in passages 6 and 10 to be 64.85% and 54.39%, respectively, whereas abnormal DNA content (DNA aneuploidy) in these cells was 35.15% and 45.61% respectively. These finding might raise the suggestion of tumorigenic potentials of MSCs specially in advanced passages, which may lead to a precancerous immortality state triggered by DNA aneuploidy-mediated mutagenic alterations in gene expression (28). As aneuploidy reflects an altered DNA profile, we demonstrated that long-term cultured MSCs exhibited high rates of DNA aneuploidy. This finding may indicate that enhanced DNA transcription and RNA translation processes can occur in early passages of AD-MSCs. Profound DNA aneuploidy-based mutagenic changes associated with reduced proliferation potential were specially observed in advanced passages of MSCs in vitro culture, suggesting that the cells remaining in the G0/G1 phase can display a reduced ability to proliferate and migrate and can be susceptible to replicative senescence through retardation/slowness or the inhibition of cell cycle progression (29). Cells grown for long periods enhanced stressful culture conditions and might precipitate to DNA aneuploidy (e.g., trisomy or monosomy) and polyploidy-induced changes in gene expression, which might contribute to an elevated incidence of alterations in the cellular phenotype. Moreover, severe pathocytophysiological disorders, DNA rearrangements, chromosomal deletions and structural mutagenic genome abnormalities might induce oncogenic transformation in these cells (30).
Flow cytometry analysis might be considered as a good reproducible and objective determinant of the biological behavior and cytophysiological characteristics of tumors (8,31). It might be considered as an accurate measurement and quantification of DNA-ploidy modes. Thus, higher prevalence of cellular DNA aneuploidy was considered one of the most important pathophysiological symptoms.
This technique has been performed on various premalignancy-related cellular and tissue alterations (31). These results indicate that the appearance of abnormal (aneuploid) DNA may be associated with malignant carcinogenesis. Both the estimation of DNA euploidy/aneuploidy levels and calculation of the range of ratios of cell fractions residing in the different cell cycle phases have been considered indicators for metastatic neoplasms and also patient survival rates for tumours, in addition it is correlated with the clinical features of preneoplastic cases (32).
In terms of the possible use of advanced passages of MSCs in clinical trials (33-35) we propose that cytogenetic analysis using flow cytometry as well as gene expression using qPCR are considered useful and objective methods for tracking the malignancy possibilities of in vitro MSCs long-term cultivation. Moreover, the results of the current investigation indicated that the stress induced by long-term MSCs culture altered their DNA stability and remarkably increased the percentage of DNA aneuploid cells. In contrast, such dependence was not demonstrated in early MSCs passages (P1–P3).
Since genomic instability of MSCs enables them to gain characteristics like tumor cells, it is an important issue for their clinical application. Because the DNA analysis is fundamental for proving the safety of MSCs, the assessing of a cell cycle and DNA structure are efficient indicators of genetic firmness of MSCs (36).
Using flow cytometry for cytometric estimation of the degree of DNA content aberration is considered the best criterion of all cytogenetic techniques. It has been reported that BM-MSCs at early passages (P3–P4) had a normal DNA content without clonal aberrations, that indicates their potential use in a clinical application, as shown before (6). However, MSCs using late passages (P6–P7) human BM-MSCs exhibited signs of senescence; gradually decreased growth, and enlarged flattened morphology (37). Cellular senescence is manifested by irreversible cell cycle arrest which is known for premature senescence associated with stress (7).
To determine the effect of long-term MSCs in vitro cultivation on nuclear DNA profile and cell cycle distribution, the distribution of DNA diploidy and aneuploidy was estimated. This determination might be helpful to check the genomic stability of our MSCs and accurate determining the safest passage and/or passages for potential use in clinical applications. To estimate aneuploidy/diploidy and analyze the cell cycle, flow cytometry was applied while real-time PCR was used for periostin gene expression quantification.
Using chi-squared test, we compared the cells with diploid and aneuploid DNA in different phases and the DNA indices between ten passages of AD-MSCs. At passage 5, we observed a considerable increase in the percentage of cells having an aneuploid DNA stem line and a remarkable reduction of DNA stability; however, a similar observation was not found in the early passages (P1–P4) of MSCs. Furthermore, the DI and the percentage of cells in each phase were calculated. The expression of periostin was up-regulated in late MSC passages (P7 onwards).
FCM is routinely used in determination of surface marker expression patterns for cell-type classification, but MSCs, based on a single marker protein expression, cannot be detected, due to their heterogeneous characteristics thus consecutive single-parameter measurements could not help a lot for determination of various stem cell markers. Therefore, to define human MSCs proposed by Committee of the International Society for Cellular Therapy, three characters are reported: (I) MSCs adhered when cultured in standard conditions; (II) MSCs must express CD90, CD73, and CD105, and not express CD34 and CD45 surface antigens; (III) MSCs must differentiate in vitro into chondroblasts, osteoblasts and adipocytes (11). In the present study, we observed in the early passages, that CD105, CD73 and CD90 were expressed by more than 99% of the cells, with only 8.7% of the cells expressed CD34. However, early reduction of CD105 marker at P5, meanwhile increased expression of CD34 in the same passage was observed. Although other markers which expressed in early passages, CD73 and CD90, remained stable till P7, then their expression began to decrease at P8.
Interestingly, immunophenotype surface marker expression might be used in differential manifestation of the onset of senescence in AD-MSC long-term cultivation. We observed that AD-MSCs enter senescence after P4 as manifested by reduction of CD105 expression at P5 meanwhile increased expression of CD34, which was not expressed before, in the same passage. Similar observation was reported by Somasundaram et al. Moreover, they reported that part of non-proliferating MSC population gained the expression of negative markers and lost the expression of positive markers in P7 (38). Also, Wagner et al. (39) observed that surface marker expression was affected by in vitro cultivation. Surface antigen detection in early passages, when compared to advanced passages, was much higher. Thus, by using cell-surface markers, identification of senescent MSCs is not a reliable method and could not be considered alone for evaluation of safety as well as efficiency of MSCs in clinical use.
Proliferation is an essential character of stem cells important for expansion, self-renewal and stemness degree defining (25). PDT is a good way to assess cell proliferation and is recommended to describe the time for cells in culture by the Cell Products Working Party (EMA) (40). At early passages, we showed that AD-MSCs were highly proliferative and maintained a fibroblast-like morphology or spindle-shaped, typical for adult AD-MSCs. Furthermore, they exhibited immunophenotype in accordance with ISCT guidelines (11). By expanding AD-MSCs (until P10) we were trying to investigate the possibility for additional clinically relevant amounts of cells. However, their growth gradually decreased in the late passages and they indicated senescence by acquiring an enlarged flattened morphology (37). Irreversible cell cycle arrest is a characteristic feature of cellular senescence (7).
From this study, it was concluded that long-term cultivation of human AD-MSCs changed their characters in an aberrant way. Appearance of aneuploidy changes in DI assay by flow cytometry in passage 5 is an indicator of beginning of mutagenic changes in these cells. Significant increase in periostin gene expression was observed with delayed onset in comparison to that of DI. Since, there is reduction of mesenchymal characters of AD-MSCs which might be implicated in the reduction of their tissue regenerative efficiency, the first four passages might be the most appropriate passages for therapy. More research is still needed to maintain the stemness characters of in vitro cultured MSCs, like studying the possible protective effect of some anti-aging factors like resveratrol during their in vitro cultivation. More investigation and understanding of the variations associated with their in vitro cultivation are needed to help in standardizing the expansion of MSCs-based therapies.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures have been approved by the Medical Ethics Committee, University of Assiut (No. E2017MED181). Written and verbal informed consent was obtained from all donors, who were free from HBV and HIV and non-smokers, prior to enrollment in the study.
References
- Bochev I, Elmadjian G, Kyurkchiev D, et al. Mesenchymal stem cells from human bone marrow or adipose tissue differently modulate mitogen-stimulated B-cell immunoglobulin production in vitro. Cell Biol Int 2008;32:384-93. [Crossref] [PubMed]
- Chung MT, Zimmermann AS, Paik KJ, et al. Isolation of human adipose-derived stromal cells using laser-assisted liposuction and their therapeutic potential in regenerative medicine. Stem Cells Transl Med 2013;2:808-17. [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Kim SY, Im K, Park SN, et al. Asymmetric aneuploidy in mesenchymal stromal cells detected by in situ karyotyping and fluorescence in situ hybridization: suggestions for reference values for stem cells. Stem Cells Dev 2015;24:77-92. [Crossref] [PubMed]
- Wang Y, Huso DL, Harrington J, et al. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy 2005;7:509-19. [Crossref] [PubMed]
- Jones M, Varella-Garcia M, Skokan M, et al. Genetic stability of bone marrow-derived human mesenchymal stromal cells in the Quantum System. Cytotherapy 2013;15:1323-39. [Crossref] [PubMed]
- Ohtani N, Hara E. Roles and mechanisms of cellular senescence in regulation of tissue homeostasis. Cancer Sci 2013;104:525-30. [Crossref] [PubMed]
- Rubio D, Garcia-Castro J, Martin MC, et al. Spontaneous human adult stem cell transformation. Cancer Res 2005;65:3035-9. [Crossref] [PubMed]
- Ghatak S, Misra S, Norris RA, et al. Periostin induces intracellular cross-talk between kinases and hyaluronan in atrioventricular valvulogenesis. J Biol Chem 2014;289:8545-61. [Crossref] [PubMed]
- Wang Y, Han ZB, Song YP, et al. Safety of mesenchymal stem cells for clinical application. Stem Cells Int 2012;2012:652034.
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315-7. [Crossref] [PubMed]
- Torre ML, Lucarelli E, Guidi S, et al. Ex vivo expanded mesenchymal stromal cell minimal quality requirements for clinical application. Stem Cells Dev 2015;24:677-85. [Crossref] [PubMed]
- Bayati V, Hashemitabar M, Gazor R, et al. Expression of surface markers and myogenic potential of rat bone marrow- and adipose-derived stem cells: a comparative study. Anat Cell Biol 2013;46:113-21. [Crossref] [PubMed]
- Lotfy A, Salama M, Zahran F, et al. Characterization of mesenchymal stem cells derived from rat bone marrow and adipose tissue: a comparative study. Int J Stem Cells 2014;7:135-42. [Crossref] [PubMed]
- Opiela J, Samiec M, Bochenek M, et al. DNA aneuploidy in porcine bone marrow-derived mesenchymal stem cells undergoing osteogenic and adipogenic in vitro differentiation. Cell Reprogram 2013;15:425-34. [Crossref] [PubMed]
- Kundrotas G, Gasperskaja E, Slapsyte G, et al. Identity, proliferation capacity, genomic stability and novel senescence markers of mesenchymal stem cells isolated from low volume of human bone marrow. Oncotarget 2016;7:10788-802. [Crossref] [PubMed]
- Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem 2001;82:583-90. [Crossref] [PubMed]
- Nejadnik H, Hui JH, Feng Choong EP, et al. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med 2010;38:1110-6. [Crossref] [PubMed]
- Higuchi O, Okabe M, Yoshida T, et al. Stemness of human Wharton's jelly mesenchymal cells is maintained by floating cultivation. Cell Reprogram 2012;14:448-55. [Crossref] [PubMed]
- Koike C, Zhou K, Takeda Y, et al. Characterization of amniotic stem cells. Cell Reprogram 2014;16:298-305. [Crossref] [PubMed]
- Coutu DL, Wu JH, Monette A, et al. Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J Biol Chem 2008;283:17991-8001. [Crossref] [PubMed]
- Shao R, Bao S, Bai X, et al. Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol 2004;24:3992-4003. [Crossref] [PubMed]
- Merle B, Garnero P. The multiple facets of periostin in bone metabolism. Osteoporos Int 2012;23:1199-212. [Crossref] [PubMed]
- Kudo Y, Ogawa I, Kitajima S, et al. Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res 2006;66:6928-35. [Crossref] [PubMed]
- Wang X, Liu J, Wang Z, et al. Periostin contributes to the acquisition of multipotent stem cell-like properties in human mammary epithelial cells and breast cancer cells. PLoS One 2013;8:e72962. [Crossref] [PubMed]
- Wagner W, Bork S, Lepperdinger G, et al. How to track cellular aging of mesenchymal stromal cells? Aging (Albany NY) 2010;2:224-30. [Crossref] [PubMed]
- Liu C, Feng X, Wang B, et al. Bone marrow mesenchymal stem cells promote head and neck cancer progression through Periostin-mediated phosphoinositide 3-kinase/Akt/mammalian target of rapamycin. Cancer Sci 2018;109:688-98. [Crossref] [PubMed]
- Tang L, Yin Y, Zhou H, et al. Proliferative capacity and pluripotent characteristics of porcine adult stem cells derived from adipose tissue and bone marrow. Cell Reprogram 2012;14:342-52. [Crossref] [PubMed]
- Bork S, Pfister S, Witt H, et al. DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell 2010;9:54-63. [Crossref] [PubMed]
- Qin Y, Ji H, Wu Y, et al. Chromosomal instability of murine adipose tissue-derived mesenchymal stem cells in long-term culture and development of cloned embryos. Cloning Stem Cells 2009;11:445-52. [Crossref] [PubMed]
- Nunez R. DNA measurement and cell cycle analysis by flow cytometry. Curr Issues Mol Biol 2001;3:67-70. [PubMed]
- Kimball RE, Schlaerth JB, Kute TE, et al. Flow cytometric analysis of lymph node metastases in advanced ovarian cancer: clinical and biologic significance. Am J Obstet Gynecol 1997;176:1319-26; discussion 1326-7. [Crossref] [PubMed]
- Motlik J, Klima J, Dvorankova B, et al. Porcine epidermal stem cells as a biomedical model for wound healing and normal/malignant epithelial cell propagation. Theriogenology 2007;67:105-11. [Crossref] [PubMed]
- Nagura S, Otaka S, Koike C, et al. Effect of exogenous Oct4 overexpression on cardiomyocyte differentiation of human amniotic mesenchymal cells. Cell Reprogram 2013;15:471-80. [Crossref] [PubMed]
- Otaka S, Nagura S, Koike C, et al. Selective isolation of nanog-positive human amniotic mesenchymal cells and differentiation into cardiomyocytes. Cell Reprogram 2013;15:80-91. [Crossref] [PubMed]
- Sensebe L. Beyond genetic stability of mesenchymal stromal cells. Cytotherapy 2013;15:1307-8. [Crossref] [PubMed]
- Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev 2006;5:91-116. [Crossref] [PubMed]
- Somasundaram I, Mishra R, Radhakrishnan H, et al. Human adult stem cells maintain a constant phenotype profile irrespective of their origin, Basal media, and long term cultures. Stem Cells Int 2015;2015:146051.
- Wagner W, Horn P, Castoldi M, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One 2008;3:e2213. [Crossref] [PubMed]
- Barkholt L, Flory E, Jekerle V, et al. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy 2013;15:753-9. [Crossref] [PubMed]
Cite this article as: Saad Eldien HM, Abdel-Aziz HO, Sayed D, Mubarak W, Hareedy HH, Mansor SG, Yoshida T, Fathy M. Periostin expression and characters of human adipose tissue-derived mesenchymal stromal cells were aberrantly affected by in vitro cultivation. Stem Cell Investig 2019;6:33.


