
Isolating human dermal fibroblasts using serial explant culture
Introduction
Tissue engineering has thus far been regarded as a fascinating field in medical research and clinical trials, since it is able to replace damaged tissues using engineered ones and it has also applications in regenerative medicine. One of tissues placed in this area is the skin, which has been already investigated in numerous studies. Although various forms of the skin substitutes can be applied and given abundant experimental studies have been carried out in this respect, there is still much more works to do in this field, especially in terms of using induction methods for optimal inducers. The main cells of the skin dermis layer are known as fibroblasts, which can be applied in tissue engineering and regenerative medicine. Some studies have also reported that, a totipotent stem cell endowed with an effective inducer is necessary for acceptable designing of engineered tissues (1-5).
Dermal fibroblasts can be involved in a wide variety of critical functions such as maintaining and protecting the skin morphology and physiology including synthesis and secretion of collagen as well as many extracellular matrix (ECM) molecules (6). In addition, such functions are of utmost importance in many other tissues and thus dermal fibroblasts can be taken into account as an available and strong cell for regenerative medicine and tissue engineering (7).
The skin dermis, enriched with fibroblasts, can further play a significant role in the growth of other cells. Therefore; the skin is considered as an appropriate tissue, applied as a source of human fibroblasts. It also has various functions in supporting related tissues and such potentials can be utilized in clinical applications including synthesizing and secreting ECM molecules as well as generating a complex mixture of bioactive factors, which can consequently regulate wound healing and immune responses (8).
In this regard, explant outgrowth and enzymatic procedures have been recognized as two important techniques that are currently being used to obtain fibroblasts from the human skin biopsies (9-15). Some studies in this field have also reported that the fibroblasts in enzymatic method can be readily released and the minimum of cells grow properly (9,10,13). Likewise, it has been frequently demonstrated that isolation of fibroblasts from different samples is time-consuming and requires a lot of materials (11,14,15). Therefore; given the disadvantages of the current methods, it is essential to improve isolation and culture techniques of human dermal fibroblasts (HDFs) to achieve an optimized procedure for medical applications.
Accordingly, the present study was to establish an improved isolation method for fibroblasts characterized by shorter time and lower costs as well as acquisition of more cells in limited enzymatic incubation time from a small piece of samples for regenerative medicine and tissue engineering applications. This study presented a modified serial explant culture as a technique with efficiency that could be applied in research and medical fields.
Methods
Materials
To conduct this study; DMEM/F12 medium, penicillin and streptomycin, gentamicin, L-glutamine, collagenase I, and amphotericin were acquired from GIBCO (NY, the United States). Moreover; bovine serum albumin (BSA), paraformaldehyde, 3-(4, 5-dimethyl) thiazol-2-yl-2, 5-dimethyl tetrazolium bromide (MTT), goat anti-mouse FITC-conjugated secondary antibody, and KCl solution were purchased from Sigma-Aldrich Corporation (NY, the United States). As well, triton X-100 was acquired from Merck & Co., Inc (NJ, the United States). Monoclonal antibodies against anti-cytokeratin, anti-vimentin, and anti-fibronectin were also purchased from Abcam Inc. (Cambridge, MA, the United States). As well, KaryoMAX Colcemid solution was acquired from Invitrogen (the United States).
Isolating HDFs via serial explant culture
Human skin specimens were obtained from 6 male individuals (age range of 20–50 years) out of healthy ones admitted for regenerative surgery. The size of skin sample was about 2×2 cm2 and the protocol approved by Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IRB number: IR.AJUMS.REC.1394.657), Ahvaz, Iran, through obtaining an informed consent from patients referred to Imam Khomeini Hospital of Ahvaz, Iran. The skin samples were kept in the culture medium on ice for transportation. The given medium contained DMEM with 0.5 µg/mL amphotericin B, 100 IU/mL penicillin, 100 IU/mL gentamycin, and 100 µg/mL streptomycin. The procedure used for cell isolation started quickly in the cell culture room of the Cellular and Molecular Research Center affiliated with Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. The samples were then sterilized for 10 seconds in 70% ethanol and rinsed 3 times with sterile PBS. The blood vessels and the whole hypodermal adipose tissues were subsequently removed and discarded. The samples were cut into 3×4 mm2 pieces and then digested by means of 0.3% collagenase I in 37 °C for 0.5 h using gentle shakes to allow the fibroblasts migrate more efficiently out of the explants once cultured onto a Petri dish. Afterwards, each piece of the undigested dermis was placed in one drop of medium and explanted onto the specified petri dishes. The medium contained DMEM/F12, 100 IU/mL penicillin, 20% fetal bovine serum (FBS), 25 µg/mL gentamycin, 100 µg/mL streptomycin, 1 µg/mL amphotericin B, and 2 mM L-glutamine. On the next day, cells began to grow out of the explants; and on the subsequent days, the explants were serially cultured into the new petri dishes until day 10. Consequently, the cells in the petri dishes were maintained in such a condition until they became confluent. To observe the normal growth, the given cells were also inspected on a daily basis (Figure 1).

Immunofluorescence (IF)
Using 4% paraformaldehyde, the cells were fixed for 20 min. Then, they were rinsed with PBS, and incubated for 10 min with Triton X-100. After that, the fixed cells were rinsed and incubated for 2 h via 3% BSA to block non-specific binding. The cells were also incubated using primary antibodies for 2 h at 4 °C. The following primary antibodies were applied in this respect including monoclonal anti-vimentin (1:100), monoclonal anti-fibronectin (1:500), and monoclonal anti-cytokeratin (1:1,000). Then, the cells were rinsed 3 times with PBS and subsequently incubated for 1 h via goat anti-mouse FITC-conjugated secondary antibody (1:150).
Nuclear staining was also performed at room temperature by means of DAPI (1:400) for 15 min. Finally, the cells were rinsed 3 times through PBS and they were observed using invert fluorescent standard microscope (IX 71, Olympus, Japan). The corresponding negative controls were also set by omitting the primary antibody within the first step to confirm lack of any non-specific binding.
Karyotyping
Karyotyping via Giemsa-banding (G-banding) was performed on passage 20 of the HDFs. The culture medium was also replaced with the media containing 0.1 µg/mL KaryoMAX Colcemid solutions for each flask, as cell confluence reached about 60–70% in the flask; and then, the culture was restored to the CO2 incubator. After one hour, the cells were collected and suspended in7 mL of 0.075 M KCl solution and for 27 min, the suspension was incubated at 37 °C. Then, 1 mL of cold Carnoy’s Fixative (methanol/acetic acid, 3:1) was added to the suspension and mixed. The cells were centrifuged at 2,800 ×g at room temperature for 10 min and the cell pellets were subsequently collected. After two rounds of fixations (adding 5 mL fixative and centrifugation at 2,800 ×g for 10 min), the pellets were fixed in 3 mL of cold fixative for 24 h at 4 °C. Consequently, the pellets were suspended in 1 mL of cold fixative and the cells obtained from each suspension were dispersed onto glass slides and baked at 75 °C for 2 h. Routine chromosome G-banding analysis was finally carried out. Together, a total number of 20 karyotypes per slide were assessed.
Results
Serial explant culture
The steps involved in serial explant culture were depicted in Figure 1. During the first day, an early migration of the HDFs from the explants was observed and their population grew following their migration and proliferation in serial cultures. The HDFs also migrated out of the tissue explants after 24 h of being treated by collagenase I. These experiments terminated after 10 serial cultures of the explants and ultimately 10 Petri dishes of the cells were harvested. After 10 days, it seemed that most of the HDFs had migrated out of the explants. During each 24 h about (1–1.5) ×106 cell was also released and ~10–15 million cells were finally obtained after 10 days. Moreover, tissue explants were mechanically dispersed and the cells were also spread and settled down. Following the use of serial explant culture, an increasing number of the HDFs had also migrated, the explants had been completely digested, and no debris of dermal tissues had remained after 10 days. Microscopic illustrations of the HDFs on different days were presented in Figure 2.
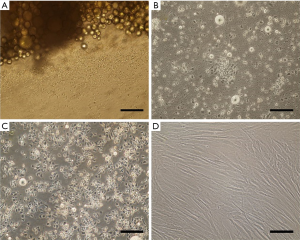
Protein expression
Expression of specific markers in the HDFs as they grew out through the explants was examined via IF. Staining was also carried out as soon as the cells reached 50% confluence. The cells were then immunostained for vimentin and fibronectin as fibroblast specific markers and cytokeratin as a standard marker for epidermal cells. The fibroblasts presented a strong and positive immunoreactivity for the vimentin and fibronectin markers (Figure 2B,D and Figure 3). In this respect; no positive immunoreactivity was observed for cytokeratin; the DAPI staining also presented proper, sharp, and blue nuclei of the growing HDFs (Figure 3).

Karyotyping
The passage 20 of the HDFs was assessed using G-banding in order to determine the genetic stability of their subculture. Karyotypes per slide were also analyzed and the HDFs expressed a normal karyotype (20/20, 46 chromosomes, XY) as illustrated in Figure 4. Abnormalities such as insertions, deletions, and duplications were not observed in passage 20 of the HDFs in the G-banding analysis.

Discussion
In the present study, a modified version of explant technique known as serial explant culture was developed for primary fibroblast cells derived from the human skin.
It should be noted that fibroblasts are the most abundant cells in the human body and their main role is to produce fibers and components of the ECM in connective tissues (1,2). Since they promptly proliferate under culture conditions, they have been widely applied in numerous in vitro studies. Fibroblasts have been also widely employed as a potential source for somatic cells in genetic manipulations as well as tissue engineering (16). Moreover, procedures concerning isolation and culture of primary fibroblasts have been proposed for decades, although reports of improvements in such techniques have been rare and they have not been highly effective (13,14). Most standard methods have been single/mixtures of enzymes and explant culture for fibroblast isolation (9-15).
As well, digestion is currently performed using a variety of matrix metalloproteinase, particularly collagenases (10,14,17). Moreover, enzyme digestion can quickly generate a great number of fibroblasts, but the main problem is incomplete tissue digestion that may be observed whenever a small amount of tissue is processed and the main part should be discarded (14,18).
In explant technique, a small piece of skin can be settled on a culture dish and produce a sizable outgrowth of cells. This technique has long been also employed as a model of wound healing (9,19,20). Nevertheless, the explant technique for isolation and culture of dermal fibroblasts has been thus far regarded as the slowest way to obtain the desired number of fibroblasts compared with enzyme digestion methods.
Some studies had previously found that different explants of 1 mm2 skin biopsies per 25 cm2 flask could be cultured 4 times (11,21). In other investigations, several standard protocols had been also proposed for harvesting HSFs using skin explants for up to two transfers before discarding the explant tissues (22). Even with the above-mentioned protocols in this study procedure, isolation of HDFs was evaluated for the maximum number of fibroblasts and a high-quality culture yield was obtained in a shorter time after 10 serial transfers for 10 days as well as higher numbers of isolated cells in comparison with those in the previous studies.
In the present protocol, partial digestion was carried out on the first day due to lose skin explant using collagenase I within 30 min; then, early migration phase of fibroblasts from explant was irritated and the population of the fibroblasts increased as a result of migration and proliferation.
Vimentin, as a permanent marker of fibroblasts, can also involve in a variety of important biological functions such as proliferation, cell contractility, stiffening, migration, and proliferation (23,24). Moreover, fibronectins play a major role in cell adhesion, migration, growth, and differentiation, and they are of utmost importance for processes such as embryonic development and wound healing. Previous studies have also reported that fibroblasts obtained from explant culture have expressed such specific markers (25,26). To confirm the characterization of the fibroblasts, the expression pattern of vimentin and fibronectin in isolated fibroblasts was assessed using the given technique. The high expression of both markers strongly supported the stability of the obtained fibroblasts.
Additionally, confirmation of cytogenetic stability and proliferation of these cells are assumed as vital steps for autologous graft in tissue repairs (14). In this regard, it has been reported that cytogenetic stability of these cells can be assessed in early (3,4) and late (10-15) passages using karyotyping (17,27); however, the genetic stability of the HDFs in the present study was evaluated after passage 20.
Overall, the positive points of the proposed protocol were:
- Reproducibility of small pieces of skin biopsies using this technique;
- Use of more technically simple and safe approaches compared with other methods;
- Consuming less time in comparison with other methods, since some previous studies had reported that the culture was expanded to the desired quantity of fibroblasts (15–20 million) within 4–8 weeks (11);
- Significant numbers of cells could be obtained from small pieces of skin samples;
- Samples could be treated via enzymes within a very short-time period, that was useful for keeping fibroblasts from variability.
Overall, this modified technique was recognized as an effective technique to obtain HDFs used in a shorter time period and faster compared with those in previously reported studies (9-15).
Form a clinical and practical perspective, this procedure could introduce a valuable and simple technique to quickly obtain large numbers of HDFs from small pieces of biopsies. Finally, this protocol would be useful for isolation and culture of precursor cells of HDFs, as an appropriate option for cell therapy as well as tissue engineering in a number of approaches in regenerative medicine including wound healing and skin repair methods.
Conclusions
This study presented a simple and applied procedure for isolation of fibroblast cells. Using this protocol, efficient cell numbers could be achieved for implantation and diagnosis in clinical research. In the present study; serial explant culture was appraised as a simple, short-time, cost-effective, and efficient method with valuable applications in research and medical fields.
Acknowledgments
Funding: We, hereby, would like to express our gratitude to the Research Council of Ahvaz Jundishapur University of Medical Sciences for their financial support of this project (Grant number: CMRC-9427).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol approved by Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (IRB number: IR.AJUMS.REC.1394.657), Ahvaz, Iran, through obtaining an informed consent from patients referred to Imam Khomeini Hospital of Ahvaz, Iran.
References
- Wood FM. Skin regeneration: the complexities of translation into clinical practise. Int J Biochem Cell Biol 2014;56:133-40. [Crossref] [PubMed]
- Vapniarsky N, Arzi B, Hu JC, et al. Concise Review: Human Dermis as an Autologous Source of Stem Cells for Tissue Engineering and Regenerative Medicine. Stem Cells Transl Med 2015;4:1187-98. [Crossref] [PubMed]
- Bayati V, Abbaspour MR, Dehbashi FN, et al. A dermal equivalent developed from adipose-derived stem cells and electrospun polycaprolactone matrix: an in vitro and in vivo study. Anat Sci Int 2017;92:509-20. [Crossref] [PubMed]
- Leirós GJ, Kusinsky AG, Drago H, et al. Dermal papilla cells improve the wound healing process and generate hair bud-like structures in grafted skin substitutes using hair follicle stem cells. Stem Cells Transl Med 2014;3:1209-19. [Crossref] [PubMed]
- T'joen V, Declercq H, Cornelissen M. Expansion of human embryonic stem cells: a comparative study. Cell Prolif 2011;44:462-76. [Crossref] [PubMed]
- Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface 2007;4:413-37. [Crossref] [PubMed]
- Fuchs E. Scratching the surface of skin development. Nature 2007;445:834-42. [Crossref] [PubMed]
- Wood F, Martin L, Lewis D, et al. A prospective randomised clinical pilot study to compare the effectiveness of Biobrane® synthetic wound dressing, with or without autologous cell suspension, to the local standard treatment regimen in paediatric scald injuries. Burns 2012;38:830-9. [Crossref] [PubMed]
- Orazizadeh M, Hashemitabar M, Bahramzadeh S, et al. Comparison of the enzymatic and explant methods for the culture of keratinocytes isolated from human foreskin. Biomed Rep. 2015;3:304-308. [Crossref] [PubMed]
- Hentzer B, Kobayasi T. Enzymatic liberation of viable cells of human skin. Acta Derm Venereol. 1978;58:197-202. [PubMed]
- Huschtscha LI, Napier CE, Noble JR, et al. Enhanced isolation of fibroblasts from human skin explants. Biotechniques 2012;53:239-44. [Crossref] [PubMed]
- Vangipuram M, Ting D, Kim S, et al. Skin punch biopsy explant culture for derivation of primary human fibroblasts. J Vis Exp 2013.e3779. [PubMed]
- Wang H, Van Blitterswijk CA, Bertrand-De Haas M, et al. Improved enzymatic isolation of fibroblasts for the creation of autologous skin substitutes. In Vitro Cell Dev Biol Anim 2004;40:268-77. [Crossref] [PubMed]
- Zaric M, Nikolic I, Zelen I, et al. Enhancement of dermal fibroblast isolation method. Serbian J Exp Clin Res 2015;6:65-9. [Crossref]
- Siengdee P, Klinhom S, Thitaram C, Nganvongpanit K. Isolation and culture of primary adult skin fibroblasts from the Asian elephant (Elephas maximus). PeerJ 2018;6:e4302. [Crossref] [PubMed]
- Dieckmann C, Renner R, Milkova L, et al. Regenerative medicine in dermatology: biomaterials, tissue engineering, stem cells, gene transfer and beyond. Exp Dermatol 2010;19:697-706. [Crossref] [PubMed]
- Huang HI, Wu CZ. Isolation and differentiation potential of fibroblast-like stromal cells derived from human skin. Methods Mol Biol 2012;879:465-70. [Crossref] [PubMed]
- De Falco E, Scafetta G, Napoletano C, et al. A standardized laboratory and surgical method for in vitro culture isolation and expansion of primary human Tenon's fibroblasts. Cell Tissue Bank 2013;14:277-87. [Crossref] [PubMed]
- Azandeh S, Orazizadeh M, Hashemitabar M, et al. Mixed enzymatic-explant protocol for isolation of mesenchymal stem cells from Wharton’s jelly and encapsulation in 3D culture system. J Biomed Sci Eng 2012;5:580-6. [Crossref]
- Halprin KM, Lueder M, Fusenig NE. Growth and differentiation of postembryonic mouse epidermal cells in explant cultures. J Invest Dermatol 1979;72:88-98. [Crossref] [PubMed]
- Balin AK, Fisher AJ, Anzelone M, et al. Effects of establishing cell cultures and cell culture conditions on the proliferative life span of human fibroblasts isolated from different tissues and donors of different ages. Exp Cell Res 2002;274:275-87. [Crossref] [PubMed]
- Freshney RI. Primary culture. Culture of animals cells: a manual of basic technique and specialized applications. New York: Wiley-Liss Inc., 2011:163-86.
- Wang N, Stamenovic D. Mechanics of vimentin intermediate filaments. J Muscle Res Cell Motil 2002;23:535-40. [Crossref] [PubMed]
- Guo M, Ehrlicher AJ, Mahammad S, et al. The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys J 2013;105:1562-8. [Crossref] [PubMed]
- Fernandes KJ, McKenzie IA, Mill P, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol 2004;6:1082-93. [Crossref] [PubMed]
- Ruetze M, Knauer T, Gallinat S, et al. A novel niche for skin derived precursors in non-follicular skin. J Dermatol Sci 2013;69:132-9. [Crossref] [PubMed]
- Maier AB, Westendorp RG. Relation between replicative senescence of human fibroblasts and life history characteristics. Ageing Res Rev 2009;8:237-43. [Crossref] [PubMed]
Cite this article as: Nejaddehbashi F, Bayati V, Mashali L, Hashemitabar M, Abbaspour M, Moghimipour E, Orazizadeh M. Isolating human dermal fibroblasts using serial explant culture. Stem Cell Investig 2019;6:23.


