
Combination of the stromal vascular fraction and platelet-rich plasma accelerates the wound healing process: pre-clinical study in a Sprague-Dawley rat model
Introduction
Burn injuries are serious global public health issue. The World Health Organization estimated that as many as 265,000 deaths annually are caused by burn injuries and many more individuals live with disabilities and disfigurement (1,2). Skin grafts are generally used to close wounds. This approach requires donor skin, commonly from the groin area, and leaves pain at the donor site, which takes more time to heal than the recipient site itself.
Recent technology offers new alternatives that only require fat and blood, instead of skin. The adipose-derived stromal vascular fraction (SVF) and adipose-derived stem cells (ADSCs) have been used to accelerate the wound healing process and to treat various diseases, such as osteoarthritis (3), multiple sclerosis (4), Crohn’s disease (5), conditions requiring fat grafting (6) and others (7). SVF can be isolated from human adipose tissue and transplanted to the patient within 2 hours (8). It contains many types of cells, including endothelial cells, pericytes, smooth muscle cells, stem cells, and other accessory immune and stromal cells that support angiogenesis, an important process in cell or tissue regeneration (9). However, the SVF has few MSCs (i.e., accounting for 1−10% of cells) (10). This may reduce the angiogenic potency of the SVF for wound healing. The low angiogenic potency of the SVF may be improved by its combination with platelet-rich plasma (PRP).
PRP is a concentrated plasma; it can be collected from whole blood and contains at least seven growth factors, including PDGFs, bFGF, VEGF, IGF-1, and TGF-β (11). SVF and PRP have been used to treat osteoarthritis (10), for fat grafting, and to treat vascular disease (7), but their combined effect on wound healing have not been reported. Therefore, the effects of SVF, PRP, and their combination were investigated using an animal model of second degree burn wounds.
Methods
Twenty-four male Sprague-Dawley rats of 8−12 weeks years old with body weight of 100−175 g were used. This study was conducted at the Animal Hospital of Bogor Agricultural Institute facility in compliance with the protocol that was reviewed and approved by the Health Research Ethics Committee of the University of Indonesia and Cipto Mangunkusumo Hospital (HREC-FMUI/CMH). Rats were randomized into the following four experimental groups: human PRP, SVF, PRP + SVF, and saline solution as a control (one experimental set) and divided into six sets. Six experimental sets used SVF and PRP from six different donors which were obtained from Klinik Hayandra with an informed consent and were isolated at the HayandraLab facility following a patented process, the H-Remedy isolation technique, developed by the HayandraLab (patent application number P00201603083).
PRP preparation
In brief, 24 mL of venous blood was collected in blood collection tubes containing 3.8% sodium citrate. Blood samples were centrifuged for 10 minutes at 800× g. Plasma was aspirated, collected into 15-mL tubes, and centrifuged for another 5 minutes at 1,000× g. The upper layer of plasma was discarded until 5 mL of plasma remained at the bottom, which was collected as PRP, frozen at −20 °C, and thawed before use.
SVF preparation
SVF was prepared from human fresh lipoaspirates harvested following a method developed by the HayandraLab. In brief, lipoaspirates were digested with in house tissue dissociation enzyme for 1 hour at 37 °C. After incubation, the enzyme was inactivated using Dulbecco’s Modified Eagle’s Medium containing 1,000 mg/L glucose and 4 mM
Wound healing model and transplantation
Wound healing models were prepared following the methods described by Zhang et al., (12) with modifications. After 1 week of adaptation, rats were injected intraperitoneally using ketamine and xylazine (0.1 mL/20 g of rat body weight). Hair on the back of rats was shaved and contacted for 4 seconds with 200-gram, flat, round-bottom stainless steel previously incubated at 100 °C in water for 5 minutes. Then, 500 µL of SVF in saline solution (40 million cells per rat), PRP, or SVF in PRP (40 million cells per rat) was injected intradermally to the back of rats randomly assigned to groups (6 rats per group). Rats were fed ad libitum for 1 week. After 1 week, rats were euthanized using ketamine and xylazine (0.1 mL/20 g of body weight). Skin tissues were subjected to histological analysis by hematoxylin-eosin (HE) staining. At the end of the study period, all rats were killed by the cervical dislocation technique.
Wound analysis
According to a previously described method (13), wound areas were traced in situ onto clear acetate paper and scanned. Scanned images of wounds were analyzed using an image analysis program (ImageJ; National Institute of Health, Bethesda, MD, USA) to measure the wound area. The two investigators who measured the wounds were blinded.
Wounds were examined based on color, surface features, hair growth, and wetness. Tissue regeneration was analyzed based on re-epithelization, the presence of skin appendages (hair follicles and sebaceous glands) and the structure of dermis, as determined by HE staining.
Statistical analysis
Numeric data are presented as mean ± standard deviation. Comparison of mean of each group was tested with ANOVA one-way if the data of each group was normally distributed and have equal variances. If one of the assumptions was violated, non-parametric Kruskal Wallis-Mann Whitney U test was performed. P value less than 0.05 is considered statistically significant.
Results
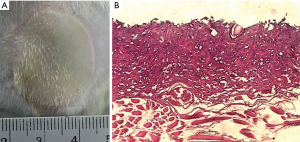
In this study, all wounds were pale with edema and delineated by a prominent ring representing the zone of hyperemia. Representative macroscopic and microscopic images obtained immediately after wounds were created are presented in Figure 1. The mean wound area was 546.26±54.20 mm2. The whole epithelial layer and reticular dermis layer were damaged in the burn group.
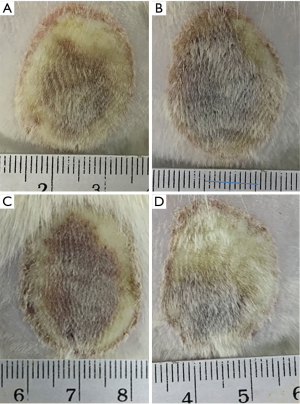
Skin exhibited swelling and started to dry after 3 days. The clinical signs on day 7 post-wound are shown in Table 1. Hyperemia was found in all groups. Only the control and PRP groups showed edema. The wound areas in the SVF, PRP, and SVF + PRP groups were almost completely dry and crusted, while most of the wound area in the control group was wet. Hair grew in all groups, but it grew faster in the SVF and SVF + PRP group than in other groups (Figure 2).

Full table
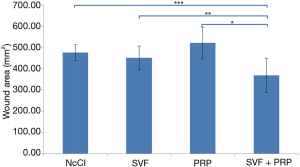
The wounds were measured on day 7 to evaluate the speed of the healing process for second degree burns. As shown in Figure 3, the mean wound area was smaller in the SVF group than in the control group, but the difference was not significant (450.38±55.08, 475.75±37.41 mm2, P=0.44). The mean wound area in the PRP group was larger than that of the control group, but this difference was also not significant (520.25±74.44 mm2, P=0.18). The mean wound area in the SVF + PRP group (368.63±80.58 mm2) was the smallest among all groups and was significantly different from those of the SVF (P=0.02), PRP (P<0.001), and control groups (P<0.001).
The biopsies of wounds from each group were analyzed by HE staining (Figure 4). Burn damage to the stratum corneum and reaching the reticular dermis layer of the skin could still be seen on day 7 post-wound in the control group. Hair follicles and sebaceous glands were present, but most skin appendages were lost in the control group. Inflammatory cells were detected in the papillary and reticular dermis.
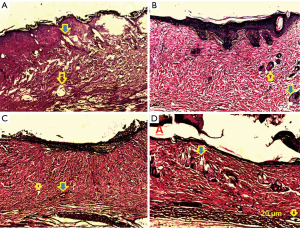
In the SVF group, the structure of the dermal layer was denser and more highly organized. The epithelial layer was thicker than that of the control group. More skin appendages and blood vessels were seen in the SVF group.
The structure of dermis was also denser and more highly organized in the PRP group. The epidermal layer in this group was thicker than that of the control group, with some detachment of the epidermis from the dermis. Signs of re-epithelization and blood vessels were seen in this group, with almost no skin appendages.
The damage on the dermal layer was diminished in the SVF + PRP group, with more skin appendages and blood vessels than in other groups. Signs of re-epithelization were clearly observed, but the epithelial layer was not completely recovered. Some detachment of the epidermis was also found in this group. Results of the histopathological evaluation for each group are presented in Table 2.

Full table
Discussion
ADSCs are used extensively to improve wound healing. Despite the ease of adipose tissue isolation, it is laborious and time-consuming to obtain sufficient quantities of ADSCs that are biologically stable for clinical use. In this study, we investigated the potency of non-expanded ADSCs, i.e., the SVF, as an alternative to approach to accelerate the wound healing process in a second degree burn wound model using Sprague-Dawley rats. Since the percentage of ADSCs in the SVF is less than 10%, the SVF was combined with PRP to improve potency.
Wound healing is complex and involves several overlapping processes. The first stage of wound healing is the inflammatory phase, in which edema occurs (14). Prolonged or excessive edema should be prevented, since it can increase the risk of tissue ischemia and infection and thereby delay the healing process (15). After 7 days, we did not observe edema in the SVF and SVF + PRP groups, while we detected minor edema in the PRP group, although the magnitude was lower than that in the control group.
Edema is defined by visible swelling of the skin caused by the uncompensated filtration of fluid from the blood to tissues due to increased vasodilatation, extravascular osmotic activity, and microvascular permeability (14). This vascular effect appears as a post-wound acute inflammatory response. The SVF contains ADSCs, which exert an anti-inflammatory effect by reducing the production of TNF-α and IL-2 and by inducing the apoptosis of activated macrophages at the transplantation site (16). Furthermore, human umbilical vein endothelial cell-ADSC co-cultures exhibit more cell-cell adherens junctions compared with those in co-cultures containing no ADSCs (17). These data indicated that treatment with ADSCs can reverse the increased vascular permeability reported in acute respiratory distress syndrome models in vivo (18).
An anti-inflammatory effect of SVF was also observed in this study, as evidenced by lower inflammatory cell infiltration in the SVF and SVF + PRP groups than in the control group. This anti-inflammatory effect was also attributed to PRP. One common marker used to investigate inflammatory responses is nitric oxide (NO) produced by phagocytes. Vascular permeability is increased by NO stimulation (19). In an in vitro osteoarthritis model, a PRP treatment group produced less NO than an untreated group (20). In another study using TNF-α as an inflammatory marker and IL-10 as an anti-inflammatory marker, TNF-α levels were significantly lower and IL-10 levels were significantly higher in the PRP group than in the untreated group (21). These results support our findings that PRP has an anti-inflammatory effect and can reduce edema.
The second phase of wound healing is the proliferative phase. In this phase, wound closure and revascularization occur via keratinocytes and fibroblasts (14). Hosni et al. reported that both MSCs and PRP promote wound closure and vascularization by increasing the production of TGF-β1 and PDGF at the transplantation site (21). A higher wound healing rate was achieved using MSCs combined with PRP than using MSC or PRP alone. Our results were similar to those obtained by Lian et al. (22).
Based on a macroscopic evaluation, SVF + PRP improved wound closure compared with SVF or PRP alone. Treatment with SVF alone reduced the wound area compared to that in the control group, but the difference was not statistically significant. Other studies have reported that PRP alone is able to improve wound closure, in contrast to the results of our study (21,22). PRP contains many growth factors (such as TGF-β1, PDGF, and VEGF) that mediate angiogenesis. These growth factors stimulate cell proliferation, migration, and capillary tube formation via many signaling pathways, such as extracellular regulated kinase (Erk) and calcineurin (CaN)/nuclear factor of activated T-cells (NFAT) signaling pathways (23-25). Differences among studies may be explained by the use of frozen-thawed PRP in this study. Roffi et al. reported that PDGF, TGF-β, and HGF are reduced during the freeze-thaw process (26). However, based on microscopic observations, all treatments (SVF, PRP, and SVF + PRP) reduced the damage in the dermal layer compared to that in the control group. In addition, we found signs of re-epithelialization in these treatment groups, but not in the control group. This suggests that PRP promotes wound healing from beneath scabs.
Revascularization also occurred in the SVF, PRP, and SVF + PRP group, as indicated by the presence of blood vessels in these treatment groups, unlike in the control group. The number of blood vessels was higher in the SVF + PRP group than in the SVF or PRP group, as were hair follicles and sebaceous glands. Hair also grew faster in the SVF + PRP group than in the groups treated with SVF or PRP alone. SVF contains many cell types, such as ADSCs, pre-adipocytes, fibroblasts, endothelial cells, macrophages, and lymphocytes (27). These cells utilize growth factors in PRP and secrete many growth factors and cytokines that modulate the microenvironment to promote wound healing (21). Many studies, including our study, support the superiority of the combination of SVF and PRP compared with SVF or PRP alone for wound healing or tissue regeneration. Given the limitations of conventional approaches for wound healing, including skin grafts, our findings have important practical implications and address an urgent clinical issue. Furthermore, our results may be of general interest to researchers interested in additive and synergistic effects of therapeutic strategies involving stem/progenitor cells. The fact that efficacy data of SVF and PRP for these diseases is limited makes the conduction of phase I-II clinical trials are required.
Conclusions
Both macroscopic and microscopic findings showed that the efficacy of SVF + PRP for wound healing was greater than those of SVF or PRP alone. The combination of SVF and PRP may provide an additive stimulatory effect that supports angiogenesis and accelerates the wound healing process.
Acknowledgments
The authors would like to thank Prof. dr. Jeanne Adiwinata Pawitan, MS, PhD and dr. Dewi Sukmawati, M.Kes., PhD from Department of Histology, Faculty of Medicine, Universitas Indonesia, Universitas Indonesia for guiding the laboratory work.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted at the Animal Hospital of Bogor Agricultural Institute facility in compliance with the protocol that was reviewed and approved by the Health Research Ethics Committee of the University of Indonesia and Cipto Mangunkusumo Hospital (HREC-FMUI/CMH).
References
- WHO. A WHO plan for burn prevention and care. WHO Document Production Services, Geneva, Switzerland, 2008.
- Bhatia A, O’Brien K, Chen M, et al. Dual therapeutic functions of F-5 fragment in burn wounds: preventing wound progression and promoting wound healing in pigs. Mol Ther Methods Clin Dev 2016;3:16041. [Crossref] [PubMed]
- Yokota N, Yamakawa M, Shirata T, et al. Clinical results following intra-articular injection of adipose-derived stromal vascular fraction cells in patients with osteoarthritis of the knee. Regen Ther 2017;6:108-12. [Crossref] [PubMed]
- Riordan NH, Ichim TE, Min WP, et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med 2009;7:29. [Crossref] [PubMed]
- Philandrianos C, Serrero M, Grimaud F, et al. First clinical case report of local microinjection of autologous fat and adipose-derived stromal vascular fraction for perianal fistula in Crohn's disease. Stem Cell Res Ther 2018;9:4. [Crossref] [PubMed]
- Gentile P, Orlandi A, Scioli MG, et al. A Comparative translational study: The combined use of enhanced stromal vascular fraction and platelet-rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cells Transl Med 2012;1:341-51. [Crossref] [PubMed]
- Comella K, Parlo M, Daly R, et al. Safety analysis of autologous stem cell therapy in a variety of degenerative diseases and injuries using the stromal vascular fraction. J Clin Med Res 2017;9:935-42. [Crossref] [PubMed]
- Gentile P, Orlandi A, Giovanna M, et al. Concise review: Adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl Med 2012;1:230-6. [Crossref] [PubMed]
- Zakhari JS, Zabonick J, Gettler B, et al. Vasculogenic and angiogenic potential of adipose stromal vascular fraction cell populations in vitro. In Vitro Cell Dev Biol Anim 2018;54:32-40. [Crossref] [PubMed]
- Van Pham P, Bui KH, Ngo DQ, et al. Transplantation of nonexpanded adipose stromal vascular fraction and platelet-rich plasma for articular cartilage injury treatment in mice model. J Med Eng 2013;2013:832396. [Crossref] [PubMed]
- Tobita M, Tajima S, Mizuno H. Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: Stem cell transplantation methods that enhance stemness. Stem Cell Res Ther 2015;6:215. [Crossref] [PubMed]
- Zhang X, Wei X, Liu L, et al. Increasing burn severity in mice is associated with delayed mobilization of circulating angiogenic cells. Arch Surg 2010;145:259-66. [Crossref] [PubMed]
- Lim JS, Yoo G. Effects of adipose-derived stromal cells and of their extract on wound healing in a mouse model. J Korean Med Sci 2010;25:746-51. [Crossref] [PubMed]
- Rowan MP, Cancio LC, Elster EA, et al. Burn wound healing and treatment: Review and advancements. Crit Care 2015;19:243. [Crossref] [PubMed]
- Armstrong DG, Nguyen HC. Improvement in healing with aggressive edema reduction after debridement of foot infection in person with diabetes. Arch Surg 2000;135:1405-9. [Crossref] [PubMed]
- Kotani T, Masutani R, Suzuka T, et al. Anti-inflammatory and anti-fibrotic effects of intravenous adipose-derived stem cell transplantation in a mouse model of bleomycin-induced interstitial pneumonia. Sci Rep 2017;7:14608. [Crossref] [PubMed]
- Grainger SJ, Putnam AJ. Assessing the permeability of engineered capillary networks in a 3d culture. PLoS One 2011;6:e22086. [Crossref] [PubMed]
- Ihara K, Fukuda S, Enkhtaivan B, et al. Adipose-derived stem cells attenuate pulmonary microvascular hyperpermeability after smoke inhalation. PLoS One 2017;12:e0185937. [Crossref] [PubMed]
- Yang B, Cai B, Deng P, et al. Nitric oxide increases arterial endotheial permeability through mediating VE-Cadherin expression during arteriogenesis. PLoS One 2015;10:e0127931. [Crossref] [PubMed]
- Osterman C, McCarthy MBR, Cote MP, et al. Platelet-rich plasma increases anti-inflammatory markers in a human coculture model for osteoarthritis. Am J Sports Med 2015;43:1474-84. [Crossref] [PubMed]
- Hosni Ahmed H, Rashed LA, Mahfouz S, et al. Can mesenchymal stem cells pretreated with platelet rich plasma modulate tissue remodeling ina rat burn? Biochem Cell Biol 2017;95:537-48. [Crossref] [PubMed]
- Lian Z, Yin X, Li H, et al. Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma instreptozotocin-induced diabetic rats. Ann Dermatol 2014;26:1-10. [Crossref] [PubMed]
- Hoeben A, Landuyt B, Highley MS, et al. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 2004;56:549-80. [Crossref] [PubMed]
- Holmes K, Chapman E, See V, et al. VEGF stimulates RCAN1.4 expression in endothelial cells via a pathway requiring Ca2+/ calcineurin and protein kinase C-delta. PLoS One 2010;5:e11435. [Crossref] [PubMed]
- Yang L, Guan H, He J, et al. VEGF increases the proliferative capacity and eNOS/NO levels of endothelial progenitor cells through the calcineurin/NFAT signalling pathway. Cell Biol Int 2012;36:21-7. [Crossref] [PubMed]
- Roffi A, Filardo G, Assirelli E, et al. Does platelet-rich plasma freeze-thawing influence growth factor release and their effects on chondrocytes and synoviocytes? Biomed Res Int 2014;2014:692913. [Crossref] [PubMed]
- Baptista L, Silva K, Pedrosa C, et al. Processing of lipoaspirate samples for optimal mesenchymal stem cells isolation, advanced techniques in liposuction and fat transfer, Nikolay Serdev. IntechOpen. 2011; DOI: 10.5772/20373. Available online: https://www.intechopen.com/books/advanced-techniques-in-liposuction-and-fat-transfer/processing-of-lipoaspirate-samples-for-optimal-mesenchymal-stem-cells-isolation [Crossref]
Cite this article as: Karina , Samudra MF, Rosadi I, Afini I, Widyastuti T, Sobariah S, Remelia M, Puspitasari RL, Rosliana I, Tunggadewi TI. Combination of the stromal vascular fraction and platelet-rich plasma accelerates the wound healing process: pre-clinical study in a Sprague-Dawley rat model. Stem Cell Investig 2019;6:18.


