
CD157 and CD200 at the crossroads of endothelial remodeling and immune regulation
The endothelial cells that coat the inner wall of blood vessels are essential for the maintenance of the vascular network, metabolic homeostasis and stem cell populations in tissue or tumor microenvironments (1-3). Angiogenesis is defined as neovascular formation through the sprouting and proliferation of endothelial cells from preexisting blood vessels. VEGF (VEGFA) and FGFs that transduce signals through VEGFR2 and FGFRs, respectively, are representative proangiogenic factors (4,5). In contrast, hematopoietic stem cells (HSCs) generated from hemangiogenic endothelial cells in the aorta-gonad-mesonephros (AGM) region of the developing embryo ultimately reside in the perivascular niche of postnatal bone marrow (6). HSC-derived myeloid progenitor cells give rise to macrophages, myeloid-derived suppressor cells (MDSCs) and endothelial progenitor cells (EPCs, also called myeloid angiogenic cells or MACs) that regulate angiogenesis and immunity (7). M2-like macrophages and MDSCs produce VEGF and FGF2 to promote angiogenesis (8), whereas EPCs integrate into the endothelial network of blood vessels to support vascular regeneration (9). Endothelial and immune cells work together in a variety of processes during fetal development, tissue repair and tumor formation.
Recently, Wakabayashi et al. found upregulation of the expression of Abcg2, Abcb1a, Cd34, Cd157 (Bst1), Cd200 (Ox2), Cxcl12, Dusp2, Igfbp3, Il6, Mycn, Sema3g and Tnfrsf10b in the stem cell-enriched “side population” of liver endothelial cells in comparison with the main population of liver endothelial cells (10). The authors focused on the surface markers CD157 and CD200 and found that CD157/CD200 double-positive liver endothelial cells formed more CD31 (PECAM1)-positive colonies than CD200 single-positive or CD157/CD200 double-negative liver endothelial cells in vitro. The expression levels of Atf3, Fosl2, Myc and Sox7 were significantly upregulated in the CD157/CD200 double-positive cells compared with the CD200 single-positive or CD157/CD200 double-negative cells; however, the functions of these transcription factors in CD157/CD200 double-positive endothelial cells remain unclear. CD157/CD200 double-positive endothelial cells derived from other organs or tissues, including the brain, heart, limb muscle, lungs, retina and skin, possess enhanced endothelial colony-forming potential. Wakabayashi et al. transplanted endothelial cells into the splenic parenchyma of adult mice after inducing endothelial damages with genotoxic pyrrolizidine alkaloid and subsequent whole-body irradiation and found that CD157/CD200 double-positive endothelial cells were incorporated into the damaged liver vasculature; gave rise to CD157/CD200 double-positive, CD200 single-positive and CD157/CD200 double-negative endothelial cells; and reconstituted the portal vein, sinusoids and central vein in the repaired liver.
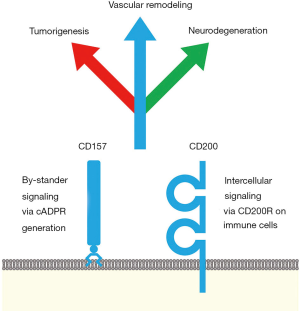
CD200 and CD200R1 are dual markers of mammary stem cells with mammosphere-forming potential and mammary gland-repopulating capacity, and the expression levels of Cd157, Cdh3, Fzd7, Lgr4, Lgr6 and Wnt10a are upregulated in the CD200/CD200R1 double-high population of mammary epithelial cells (11). CD200 is a marker of the limbal stem cells that maintain the corneal tissue, and ABCB5, CDH3, PAX6 and WNT7A expression levels are upregulated in the CD200-positive population of corneal epithelial cells (12). In contrast, CD157 is a marker of Paneth cells in intestinal crypts that support the self-renewal and proliferation of intestinal stem cells (13), as well as fibroblastic reticular cells at the interface of germinal centers and the T cell zone that support the affinity maturation of plasma cells (14). These facts indicate that CD157 and CD200 are surface markers of stem or niche cells in several tissue microenvironments.
CD157, a glycosylphosphatidylinositol (GPI)-anchored protein, functions as a component of integrin adhesion receptor complexes that activate SRC, ERK and AKT signaling cascades and as an ectoenzyme catalyzing nicotinamide adenine dinucleotide into cyclic ADP-ribose, which increases the intracellular Ca2+ concentration through mobilization from the intracellular pool (15). CD157 expression is found on neutrophils and upregulated by the chemokine CCL2 (MCP1) in circulating monocytes, and these expression patterns regulates the transendothelial migration of neutrophils and monocytes, respectively (16,17). CD157 is involved in the integrin-mediated migration of UE7T-13 cells derived from bone barrow mesenchymal stem cells (MSCs) (18). Because CD157 overexpression induces epithelial-to-mesenchymal transition (EMT) and enhances the motility and invasiveness of tumor cells, the upregulation of CD157 expression is associated with a poor prognosis in patients with epithelial ovarian cancer or biphasic malignant pleural mesothelioma (19,20). In addition, CD157 is expressed by hematological malignancies, such as the M4 and M5 subtypes of acute myeloid leukemia (AML) and B-cell precursor acute lymphoblastic leukemia (BCP-ALL) (21,22).
The CD157 gene and paralogous CD33 gene are clustered in a head-to-tail manner at human chromosome 4p15.32 (15). The single-nucleotide polymorphism rs11724635 of the human CD157 gene is associated with the risk of Parkinson’s disease in Asian, European and United States populations [odds ratio per minor allele dose =0.87 (P=2.43×10-9)], with a population-attributable risk of 7.82% (95% CI: 5.30–9.47) (23). Parkinson’s disease is a neurodegenerative disease that is characterized by motor symptoms, such as bradykinesia and resting tremor (24,25), and nonmotor symptoms, including anxiety, cognitive dysfunction, depression, hyposmia and sleep disorder (26,27). Cd157 knockout mice manifested anxiety- and depression-like symptoms (28); however, the causal link between the CD157 SNP and Parkinson’s disease remains unclear.
CD200 is a transmembrane protein with two extracellular immunoglobulin-like domains and a short cytoplasmic tail that is expressed on a variety of cells, such as B and T lymphocytes, endothelial cells, neurons and pancreatic islet cells (29,30), and whose expression is upregulated by IL4 (31). CD200 transduces signals through CD200R (CD200R1), a transmembrane protein with two extracellular immunoglobulin-like domains and a cytoplasmic NPxY motif (32,33). CD200R is expressed on myeloid-lineage immune cells (MDSCs, macrophages, monocytes, dendritic cells, basophils and eosinophils) and lymphocytic-lineage immune cells [T-helper type 2 (Th2) lymphocytes and innate lymphoid type 2 (ILC2) cells], and CD200R expression is upregulated in M2 macrophages and Th2 lymphocytes by IL4 and mediates immunosuppressive effects (32,34-36). Interaction between CD200 and CD200R leads to the phosphorylation of tyrosine 302 in the NPxY motif of CD200R, which recruits the Dok2-RasGAP complex to repress Ras-ERK signaling in myeloid cells (33). Cd200 knockout mice are prone to collagen-induced arthritis and experimental autoimmune encephalomyelitis owing to the activation and expansion of macrophages and microglial cells, respectively (37). Cd200 knockout mice are also resistant to chemically induced skin tumorigenesis owing to decreased immune tolerance (38), whereas compared with CD200- B16 melanoma cells, CD200+ B16 melanoma cells exhibit enhanced tumorigenesis owing to the expansion of myeloid-lineage cells and increased tumor angiogenesis in Cd200r knockout mice (39). CD200-CD200R signaling plays a critical role in cancers and noncancerous diseases through the regulation of immunity and angiogenesis.
In a clinical study, cell-surface CD200 expression on B lymphocytes was upregulated in 100% (n=87) of patients with B-cell chronic lymphocytic leukemia (B-CLL) compared with healthy donors (40), whereas cell-surface CD200 expression on blast cells was detected in 56% (136/244) of patients with AML (41). CD200 immunostaining is frequently detected in B-CLL (100%, n=21), hairy cell leukemia (100%, n=12), mediastinal large B-cell leukemia (100%, n=8), classical Hodgkin lymphoma (92%, 12/13) and multiple myeloma (77%, 10/13) among B-cell lymphoproliferative disorders (42). CD200 immunostaining is also detected in solid tumors, such as basal cell carcinoma (100%, n=9), papillary thyroid carcinoma (100%, n=10), gastrointestinal carcinoid tumors (95%, 78/82), pancreatic neuroendocrine tumors (93%, 56/60), Merkel cell carcinoma (84%, 125/149), small cell lung carcinoma (83%, 60/72), renal cell carcinoma (71%, 5/7) and ovarian cancer (67%, 6/9) (43). In addition, CD200 expression is upregulated in cancer-associated fibroblasts (44) and infiltrating CD4+ T lymphocytes (45) in patients with lung cancer or classical Hodgkin lymphoma, respectively. Because CD200 transduces immunosuppressive signals through CD200R on myeloid-lineage cells and T lymphocytes, CD200 expression on tumor cells, cancer-associated fibroblasts and CD4+ T lymphocytes can induce immune evasion through the expansion of M2-like macrophages and regulatory T (Treg) cells (46) and reduced infiltration of CD4+ and CD8+ T lymphocytes and natural killer cells (39) into the tumor microenvironment.
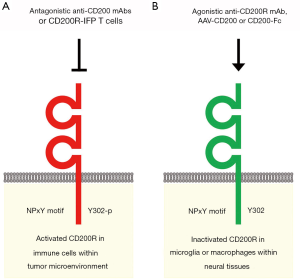
Anti-CD200 monoclonal antibodies (mAbs) (47) and engineered CD8+ T lymphocytes expressing CD200R-CD28 chimeric proteins (CD200R-IFP T cells) (48) have been developed as investigational therapeutics targeting the immunosuppressive CD200-CD200R signaling cascade. Antagonistic anti-CD200 mAbs show antitumor effects in a mouse model of B-CLL, whereas engineered chimeric CD200R-CD28 T lymphocytes showed antitumor effects in a mouse model of erythroleukemia. However, because inflammation and immune tolerance are both involved in tumorigenesis (49,50), preclinical mouse-model experiments have revealed context-dependent functions of CD200-CD200R signaling in tumor progression (39,51) and tumor suppression (52,53). The exploration of biomarkers predicting antitumor effects without severe adverse effects related to autoimmunity is necessary for the clinical application of CD200-CD200R signaling-targeted therapeutics.
Neuronal CD200 immunostaining in the central nervous system (CNS) is downregulated in the postmortem brain of patients with Alzheimer’s disease (54), multiple sclerosis (55) or Parkinson’s disease (56), which are characterized by CNS destruction mediated in part through inflammation triggered by β-amyloid, β-synuclein and α-synuclein, respectively (57-59). The defect in Cd200 expression in Cd200 knockout mice leads to enhanced microglia/macrophage activation in the CNS and accelerated neurodegeneration (37), whereas the upregulation of neuronal Cd200 expression in Wlds mice leads to decreased microglia/macrophage accumulation in the CNS and decelerated neurodegeneration (60). CD200 can protect the CNS from neurodegeneration through the maintenance of the blood-brain barrier (61), suppression of microglia/macrophage-mediated inflammation (37) and promotion of FGFR-dependent neuronal survival (62). A CD200-Fc fusion protein (63), an adeno-associated virus expressing CD200 (AAV-CD200) (64) and an agonistic anti-CD200R mAb (63) have been developed as investigational drugs that stimulate CD200 signaling for neuroprotection; however, these drugs still remain in preclinical stages.
CD157 and CD200 are surface markers of stem/niche cell populations in tissue or tumor microenvironments (Figure 1). CD157 is a GPI-anchored ectoenzyme that generates cyclic ADP-ribose and functions as an integrin-interacting protein, whereas CD200 is a transmembrane-type ligand that transduces immunosuppressive signals through CD200R. CD157 and CD200 are involved in a variety of pathophysiological processes, such as vascular regeneration, tumor progression and inflammation-related neurodegeneration (Figure 1). Anti-CD200 mAbs and CD200R-IFP T cells are investigational drugs that inhibit CD200 signaling for the treatment of cancer patients with immune evasion (Figure 2A), whereas an agonistic anti-CD200R mAb, AAV-CD200 and CD200-Fc are investigational drugs that activate CD200 signaling for the treatment of patients with neurodegenerative diseases (Figure 2B). The context-dependent functions of CD200-CD200R signaling in tumor and neuroinflammatory microenvironments should be further investigated before CD200-CD200R signaling-targeted therapeutics are applied in the clinic in the future.
Acknowledgements
This work was supported in part by a grant-in-aid from M. Katoh’s Fund for the Knowledge-Base Project.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011;473:298-307. [Crossref] [PubMed]
- Katoh M. Therapeutics targeting angiogenesis: Genetics and epigenetics, extracellular miRNAs and signaling networks. Int J Mol Med 2013;32:763-7. [Crossref] [PubMed]
- Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature 2016;529:316-25. [Crossref] [PubMed]
- Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer 2013;13:871-82. [Crossref] [PubMed]
- Katoh M. Therapeutics targeting FGF signaling network in human diseases. Trends Pharmacol Sci 2016;37:1081-96. [Crossref] [PubMed]
- Crisan M, Solaimani Kartalaei P, et al. BMP and Hedgehog regulate distinct AGM hematopoietic stem cells ex vivo. Stem Cell Reports 2016;6:383-95. [Crossref] [PubMed]
- Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer 2013;13:739-52. [Crossref] [PubMed]
- De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer 2017;17:457-74. [Crossref] [PubMed]
- Singhal M, Liu X, Inverso D, et al. Endothelial cell fitness dictates the source of regenerating liver vasculature. J Exp Med 2018;215:2497-508. [Crossref] [PubMed]
- Wakabayashi T, Naito H, Suehiro JI, et al. CD157 marks tissue-resident endothelial stem cells with homeostatic and regenerative properties. Cell Stem Cell 2018;22:384-97.e6. [Crossref] [PubMed]
- Rauner G, Kudinov T, Gilad S, et al. High Expression of CD200 and CD200R1 distinguishes stem and progenitor cell populations within mammary repopulating units. Stem Cell Reports 2018;11:288-302. [Crossref] [PubMed]
- Bojic S, Hallam D, Alcada N, et al. CD200 expression marks a population of quiescent limbal epithelial stem cells with holoclone forming ability. Stem Cells 2018;36:1723-35. [Crossref] [PubMed]
- Yilmaz ÖH, Katajisto P, Lamming DW, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 2012;486:490-5. [Crossref] [PubMed]
- Zhang Y, Tech L, George LA, et al. Plasma cell output from germinal centers is regulated by signals from Tfh and stromal cells. J Exp Med 2018;215:1227-43. [Crossref] [PubMed]
- Quarona V, Zaccarello G, Chillemi A, et al. CD38 and CD157: a long journey from activation markers to multifunctional molecules. Cytometry B Clin Cytom 2013;84:207-17. [Crossref] [PubMed]
- Ortolan E, Tibaldi EV, Ferranti B, et al. CD157 plays a pivotal role in neutrophil transendothelial migration. Blood 2006;108:4214-22. [Crossref] [PubMed]
- Lo Buono N, Parrotta R, Morone S, et al. The CD157-integrin partnership controls transendothelial migration and adhesion of human monocytes. J Biol Chem 2011;286:18681-91. [Crossref] [PubMed]
- Aomatsu E, Takahashi N, Sawada S, et al. Novel SCRG1/BST1 axis regulates self-renewal, migration, and osteogenic differentiation potential in mesenchymal stem cells. Sci Rep 2014;4:3652. [Crossref] [PubMed]
- Morone S, Lo-Buono N, Parrotta R, et al. Overexpression of CD157 contributes to epithelial ovarian cancer progression by promoting mesenchymal differentiation. PLoS One 2012;7:e43649. [Crossref] [PubMed]
- Ortolan E, Giacomino A, Martinetto F, et al. CD157 enhances malignant pleural mesothelioma aggressiveness and predicts poor clinical outcome. Oncotarget. 2014;5:6191-205. [Crossref] [PubMed]
- Krupka C, Lichtenegger FS, Köhnke T, et al. Targeting CD157 in AML using a novel, Fc-engineered antibody construct. Oncotarget 2017;8:35707-17. [Crossref] [PubMed]
- Mirkowska P, Hofmann A, Sedek L, et al. Leukemia surfaceome analysis reveals new disease-associated features. Blood 2013;121:e149-59. [Crossref] [PubMed]
- International Parkinson Disease Genomics Consortium, Nalls MA, Plagnol V, et al. Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet 2011;377:641-9. [Crossref] [PubMed]
- Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson's disease and its potential as therapeutic target. Transl Neurodegener 2015;4:19. [Crossref] [PubMed]
- Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers 2017;3:17013. [Crossref] [PubMed]
- Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci 2017;18:435-50. [Crossref] [PubMed]
- Rodríguez-Violante M, Zerón-Martínez R, Cervantes-Arriaga A, et al. Who can diagnose Parkinson's disease first? Role of pre-motor symptoms. Arch Med Res 2017;48:221-7. [Crossref] [PubMed]
- Lopatina O, Yoshihara T, Nishimura T, et al. Anxiety- and depression-like behavior in mice lacking the CD157/BST1 gene, a risk factor for Parkinson's disease. Front Behav Neurosci 2014;8:133. [Crossref] [PubMed]
- McCaughan GW, Clark MJ, Barclay AN. Characterization of the human homolog of the rat MRC OX-2 membrane glycoprotein. Immunogenetics 1987;25:329-35. [Crossref] [PubMed]
- Kelly OG, Chan MY, Martinson LA, et al. Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol 2011;29:750-6. [Crossref] [PubMed]
- Lyons A, Downer EJ, Crotty S, et al. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J Neurosci 2007;27:8309-13. [Crossref] [PubMed]
- Wright GJ, Cherwinski H, Foster-Cuevas M, et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol 2003;171:3034-46. [Crossref] [PubMed]
- Mihrshahi R, Barclay AN, Brown MH. Essential roles for Dok2 and RasGAP in CD200 receptor-mediated regulation of human myeloid cells. J Immunol 2009;183:4879-86. [Crossref] [PubMed]
- Stumpfova M, Ratner D, Desciak EB, et al. The immunosuppressive surface ligand CD200 augments the metastatic capacity of squamous cell carcinoma. Cancer Res 2010;70:2962-72. [Crossref] [PubMed]
- Koning N, van Eijk M, Pouwels W, et al. Expression of the inhibitory CD200 receptor is associated with alternative macrophage activation. J Innate Immun 2010;2:195-200. [Crossref] [PubMed]
- Blom LH, Martel BC, Larsen LF, et al. The immunoglobulin superfamily member CD200R identifies cells involved in type 2 immune responses. Allergy 2017;72:1081-90. [Crossref] [PubMed]
- Hoek RM, Ruuls SR, Murphy CA, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 2000;290:1768-71. [Crossref] [PubMed]
- Rygiel TP, Karnam G, Goverse G, et al. CD200-CD200R signaling suppresses anti-tumor responses independently of CD200 expression on the tumor. Oncogene 2012;31:2979-88. [Crossref] [PubMed]
- Liu JQ, Talebian F, Wu L, et al. A critical role for CD200R signaling in limiting the growth and metastasis of CD200+ melanoma. J Immunol 2016;197:1489-97. [Crossref] [PubMed]
- McWhirter JR, Kretz-Rommel A, Saven A, et al. Antibodies selected from combinatorial libraries block a tumor antigen that plays a key role in immunomodulation. Proc Natl Acad Sci USA 2006;103:1041-6. [Crossref] [PubMed]
- Damiani D, Tiribelli M, Raspadori D, et al. Clinical impact of CD200 expression in patients with acute myeloid leukemia and correlation with other molecular prognostic factors. Oncotarget 2015;6:30212-21. [Crossref] [PubMed]
- Dorfman DM, Shahsafaei A. CD200 (OX-2 membrane glycoprotein) expression in β cell-derived neoplasms. Am J Clin Pathol 2010;134:726-33. [Crossref] [PubMed]
- Love JE, Thompson K, Kilgore MR, et al. CD200 expression in neuroendocrine neoplasms. Am J Clin Pathol 2017;148:236-42. [Crossref] [PubMed]
- Lambrechts D, Wauters E, Boeckx B, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med 2018;24:1277-89. [Crossref] [PubMed]
- Wein F, Weniger MA, Höing B, et al. Complex immune evasion strategies in classical Hodgkin lymphoma. Cancer Immunol Res 2017;5:1122-32. [Crossref] [PubMed]
- Gaiser MR, Weis CA, Gaiser T, et al. Merkel cell carcinoma expresses the immunoregulatory ligand CD200 and induces immunosuppressive macrophages and regulatory T cells. Oncoimmunology 2018;7:e1426517. [Crossref] [PubMed]
- Kretz-Rommel A, Qin F, Dakappagari N, et al. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J Immunol 2007;178:5595-605. [Crossref] [PubMed]
- Oda SK, Daman AW, Garcia NM, et al. A CD200R-CD28 fusion protein appropriates an inhibitory signal to enhance T-cell function and therapy of murine leukemia. Blood 2017;130:2410-9. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565-70. [Crossref] [PubMed]
- Gorczynski RM, Chen Z, Khatri I, et al. Cure of metastatic growth of EMT6 tumor cells in mice following manipulation of CD200:CD200R signaling. Breast Cancer Res Treat 2013;142:271-82. [Crossref] [PubMed]
- Talebian F, Liu JQ, Liu Z, et al. Melanoma cell expression of CD200 inhibits tumor formation and lung metastasis via inhibition of myeloid cell functions. PLoS One 2012;7:e31442. [Crossref] [PubMed]
- Erin N, Podnos A, Tanriover G, et al. Bidirectional effect of CD200 on breast cancer development and metastasis, with ultimate outcome determined by tumor aggressiveness and a cancer-induced inflammatory response. Oncogene 2015;34:3860-70. [Crossref] [PubMed]
- Walker DG, Dalsing-Hernandez JE, Campbell NA, et al. Decreased expression of CD200 and CD200 receptor in Alzheimer's disease: a potential mechanism leading to chronic inflammation. Exp Neurol 2009;215:5-19. [Crossref] [PubMed]
- Koning N, Swaab DF, Hoek RM, et al. Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. J Neuropathol Exp Neurol 2009;68:159-67. [Crossref] [PubMed]
- Walker DG, Lue LF, Tang TM, et al. Changes in CD200 and intercellular adhesion molecule-1 (ICAM-1) levels in brains of Lewy body disorder cases are associated with amounts of Alzheimer's pathology not α-synuclein pathology. Neurobiol Aging 2017;54:175-86. [Crossref] [PubMed]
- Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med 2017;23:1018-27. [Crossref] [PubMed]
- Lodygin D, Hermann M, Schweingruber N, et al. β-Synuclein-reactive T cells induce autoimmune CNS grey matter degeneration. Nature 2019;566:503-8. [Crossref] [PubMed]
- Pappalardo JL, Hafler DA. Multiple sclerosis enters a grey area. Nature 2019;566:465-6. [Crossref] [PubMed]
- Chitnis T, Imitola J, Wang Y, et al. Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. Am J Pathol 2007;170:1695-712. [Crossref] [PubMed]
- Denieffe S, Kelly RJ, McDonald C, et al. Classical activation of microglia in CD200-deficient mice is a consequence of blood brain barrier permeability and infiltration of peripheral cells. Brain Behav Immun 2013;34:86-97. [Crossref] [PubMed]
- Pankratova S, Bjornsdottir H, Christensen C, et al. Immunomodulator CD200 promotes neurotrophic activity by interacting with and activating the Fibroblast Growth Factor Receptor. Mol Neurobiol 2016;53:584-94. [Crossref] [PubMed]
- Gorczynski RM, Chen Z, Lee L, et al. Anti-CD200R ameliorates collagen-induced arthritis in mice. Clin Immunol 2002;104:256-64. [Crossref] [PubMed]
- Varnum MM, Kiyota T, Ingraham KL, et al. The anti-inflammatory glycoprotein, CD200, restores neurogenesis and enhances amyloid phagocytosis in a mouse model of Alzheimer's disease. Neurobiol Aging 2015;36:2995-3007. [Crossref] [PubMed]
Cite this article as: Katoh M, Katoh M. CD157 and CD200 at the crossroads of endothelial remodeling and immune regulation. Stem Cell Investig 2019;6:10.


