
Valproic acid stimulates in vitro migration of the placenta-derived mesenchymal stem/stromal cell line CMSC29
Introduction
When cultivated in the laboratory, human primary mesenchymal stem cell (MSC) exhibits a relatively short life span and display considerable donor-to-donor variation in growth behavior. These variations make it difficult to utilize primary MSC in ex vivo optimization and experimental work. To overcome these issues, transformed cell lines are often used as they have advantages over the use of primary cells, in that they can be grown in large numbers, have a longer life span, and have more uniform growth and functional characteristics.
The CMSC29 cell line was created from human primary, term placental CMSC by human telomerase reverse transcriptase (hTERT) transformation (1). Transfected CMSC cells showed increased expression of hTERT, as expected, with no major chromosomal abnormalities (i.e., no major deletions, translocations or rearrangements) or signs of tumorigenicity in vitro (1). The characterization study also revealed that the CMSC29 cell line met accepted general criteria for MSC in terms of surface marker expression and multi-lineage differentiation potential (1). Moreover, CMSC29 were confirmed to be of fetal origin. In addition, the migratory capacity of CMSC29 was examined and compared to their primary CMSC counterparts. Using an in vitro scratch assay, there was no significant difference in the percentage of scratch closure between the primary CMSC and the cell line.
The ability of MSCs to efficiently migrate to the site of injury is still a challenge in cell-based therapy. In stem cell research, many studies are dedicated to understanding the migratory behavior of MSCs, and how to increase their therapeutic potential by increasing their tissue targeting capacity. Thus, the aim of this study was to examine CMSC29 cell migration behavior using a real-time, quantitative assay system (xCELLigence, see below) to further characterize this novel cell line. CMSC29 cell migration was assessed using two chemotactic factors; stromal cell-derived factor-1α (SDF-1α) and hepatocyte growth factor (HGF). This was done to determine whether the cell line would mimic the migration pattern of primary CMSC. Both SDF-1α and HGF are strong chemoattractants for MSCs (2-9). Abumaree et al. [2013] reported that primary CMSCs express mRNA for SDF-1α and HGF, and their receptors CXCR4 and c-met respectively. Moreover, SDF-1α and HGF significantly increase primary CMSC migration in a Transwell assay (10).
CMSC29 cell migration was also assessed using valproic acid (VPA) (2-propylpentanoic acid sodium); a histone deacetylase inhibitor (11). VPA treatment of cells was reported to increase migration via different mechanisms. In one migration study, histone deacetylase inhibition was the mechanism by which VPA stimulated bone marrow MSC (BMMSC) migration towards an SDF-1α gradient (12). CXCR4 surface expression is reduced during ex vivo MSC expansion, leaving little or no CXCR4 on the cell surface (4,13-15). Thus, most of the CXCR4 expressed by cultured MSC is likely to be located internally (4,14,15). In the mentioned BMMSC migration study, VPA caused hyperacetylation of histones, which in turn promoted a more transcriptionally active chromatin structure that increased CXCR4 gene expression and consequentially increased chemoattraction towards SDF-1α (12). Whereas another BMMSC migration study reported another mechanism of action, where VPA increased cell migration by increasing their release of trophic factors (16).
In this study, we investigated whether CMSC29 cells migrated toward the chemoattractants SDF-1α and HGF. We also investigated whether VPA treatment of CMSC29 cells increased their migration towards a chemoattractant and serum free medium.
Methods
Cell line culture, passaging and storage
MSC29 cells were cultured in AmnioMAXTM C-100 Basal Medium supplemented with 10:1 (v/v) AmnioMAXTM C-100 Supplement (Life Technologies, Carlsbad, California, USA) and kept in a humidified incubator at 37 °C, 5% CO2 and 95% room air. Cells were passaged by adding 37 °C warm TrypLETM (Life Technologies), sufficient to cover the surface area of the plate and incubated for 5 to 10 minutes, followed by deactivation with FCS. Cells that had lifted from the flask were counted and the appropriate number transferred to a fresh flask. For storage, cells were harvested, centrifuged, and resuspended in α-MEM (Life Technologies), FCS and dimethyl sulfoxide (DMSO) (6:3:1, v/v/v). The cells where transferred to a CryoTube vial (Thermo Electron Co., Waltham, Massachusetts) and the vial placed in a Nalgene Mr. FrostyTM container (−1 °C/minute cooling system from Thermo Electron Co.) overnight at −80 °C before transferring into liquid nitrogen for long term storage.
xCELLigence cell migration assay
The xCELLigence Real-Time Cell Analyzer (RTCA) DP (ACEA Biosciences, San Diego, California, USA) real-time functional assay system was used to measure cell migration. Experiments using the CIM-plate 16 (ACEA Biosciences) were carried out under sterile conditions. Wells of the lower chamber were filled with 160 µL of designated medium. The upper chamber was then placed onto the lower chamber and 50 µL of designated medium was added to wells of the upper chamber. The CIM-plate 16 was then connected to the RTCA DP analyser inside a tissue culture incubator (37 °C, 5% CO2 in air) and left for an hour to allow the membrane surface to equilibrate with the medium. The background reading (Z0) was then taken and the CIM-plate 16 was removed from the incubator. Next, 2×104 CMSC29 cells suspended in 100µl of designated medium were added to each well (cell seeding number was based on previous optimization experiments and fell within the manufacture’s recommended range) and left to settle at room temperature for 30 minutes. Finally, the CIM-plate 16 was placed into the RTCA DP analyzer and impedance measurements commenced for up to 48 hours. At each time point, impedance is measured (Zi), and cell index (CI) is then calculated based on the formula; CI = (Zi − Z0)/15 Ω. This was done to investigate the relative change in the cell index values between different test groups over time.
xCELLigence cell proliferation assay
Preparations for experiments using the E plate-16 (ACEA Biosciences) were carried out under sterile conditions. All wells were filled with 100 µL of α-MEM medium with or without VPA (Sigma-Aldrich, St. Louis, Missouri, USA). The E-plate16 was then left to sit at room temperature for 30 minutes to allow the electrode to reach equilibrium with the medium. The plate was then connected to the RTCA DP analyser and the background reading was taken (Z0). Next, 1×104 CMSC29 cells suspended in 100 µL of α-MEM medium, with or without VPA, were added to each well (cell seeding number was based on previous optimization experiments and fell within the manufacture’s recommended range), and left to sit at room temperature inside the tissue culture hood for 30 minutes to allow the cells to settle. Finally, the E plate-16 was placed on the RTCA DP analyzer and impedance measurements (Zi) commenced for up to 48 hours, with CI values calculated at each time point using the formula above.
Flow cytometric analysis of CXCR4 surface expression
CXCR4 Mouse Anti-Human, PE conjugate (R&D Systems, Minneapolis, Minnesota, USA) was used to detect CXCR4 surface expression on CMSC29 cells, and Mouse Anti-Human IgG1, PE conjugate, isotype control (BD Bioscience, Franklin Lakes, New Jersey, USA) was used to confirm that the antibody binding was specific. 1×105 cells resuspended in 1ml 2% FCS [v/v in Hank’s Balanced Salt Solution, no calcium, no magnesium, HBSS (−)] were added to each Eppendorf tube. The tubes were centrifuged and 900 µL of the supernatant was removed. Then, 5 µL of human serum (human male AB plasma, USA origin, sterile-filtered, Sigma-Aldrich) together with 1 µL of the appropriate antibody was added and the tubes were incubated in the dark at 4 °C for 30 minutes. Following incubation, the cell suspension was topped up with 900 µL 2% (v/v) FCS in HBSS (−), and centrifuged. Finally, 5 µL of DAPI (4’,6-diamidino-2-phenylindole, dihydrochloride, Sigma-Aldrich) was added along with 500 µL of 2% (v/v) FCS in HBSS (−) to all tubes, and the cell suspensions were filtered through a filter cap tube. The samples were processed in the LSR II flow cytometer (BDTM Biosciences, Franklin Lakes, New Jersey, USA) and the data were analysed using the BD FACSDiva software (BDTM Biosciences).
Statistical analysis
Data analysis was performed with Minitab 17 Statistical Software (Penn State College, PA; Minitab, Inc. www.minitab.com). Statistical significance was defined as P<0.05, and was determined by an ANOVA general linear model statistical test followed by Tukey’s HSD pairwise comparisons. The experimental replicates (n) were included as explanatory variables in all analyses.
Results
CMSC29 cell migration
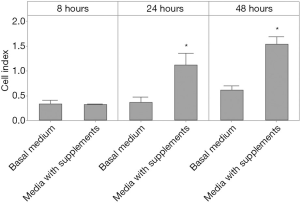
To ensure that the CMSC29 cell line was capable of responding to stimulation, supplemented CMSC29 culture medium was tested as a chemoattractant. The lower wells of the CIM-plate 16 were divided into two groups, each group in triplicate. The first group contained basal cell culture medium alone, AmnioMAXTM. The second group contained AmnioMAXTM cell culture medium with AmnioMAXTM cell growth supplements. CMSC29 cells were suspended in α-MEM medium, and added to the wells of the upper chamber. There was a significant increase in the mean cell index values at 24 and 48 hours in wells with supplemented medium compared with wells containing basal medium alone (Figure 1). This indicates that CMSC29 cells had the ability to increase their migration in response to a stimulus.
The effect of SDF-1α on CMSC29 cell migration
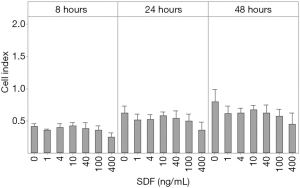
Wells of the CIM-plate 16 lower chamber were filled in duplicates with α-MEM medium containing 0, 1, 4, 10, 40, 100 or 400 ng/mL SDF-1α (R&D systems). CMSC29 cells suspended in α-MEM medium were then added to the wells of the upper chamber. The cell index values were recorded over 48 hours. Statistical analysis showed that at 8, 24 and 48 hours, SDF-1α did not stimulate migration at any of the tested concentrations (Figure 2).
The effect of HGF on CMSC29 cell migration
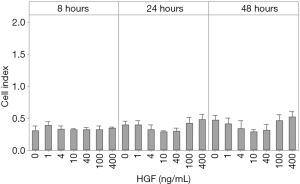
Wells of the lower chamber were filled in duplicate with α-MEM medium containing 0, 1, 4, 10, 40, 100, 200 or 400 ng/mL HGF (MACS, Bergisch Gladbach, Germany), while CMSC29 cells suspended in α-MEM medium were added to the wells of the upper chamber. Results showed that HGF did not stimulate CMSC29 cell migration at any of the tested concentrations over a 48 hour incubation period (Figure 3).
CXCR4 surface expression and the effect of VPA treatment on CMSC29 proliferation and migration towards SDF-1α
Surface expression of SDF-1α receptor, CXCR4, is reported to be internalized during in vitro expansion of cells (14,15). Treating the cells with a histone deacetylase inhibitor such as VPA, was reported to translocate CXCR4 to the cell surface and thereby increase migration towards SDF-1α (12). First, flow cytometric analysis was carried out to detect CXCR4 surface expression on CMSC29 cells. The percentage of CMSC29 cells that expressed CXCR4 was 0.18%±0.06 SEM (Figure 4).

Second, a proliferation assay was carried out to determine if VPA treatment would affect CMSC29 viability and proliferation. Increasing concentrations of VPA were tested, up to 2,500 ng/mL, in order to reach an inducing dose. CMSC29 cells were suspended in supplemented α-MEM medium containing 0-250-500-1,000-2,500 ng/mL VPA, and then seeded into wells with each concentration tested in duplicate. The control was cells suspended in the same medium without added VPA. Statistical analysis showed that following 8 and 24 hours, VPA did not affect cell viability and proliferation at any of the tested concentrations. At 48 hours, only cells that were treated with 2,500 ng/mL VPA had significant decrease in cell index values compared with the control, while the other tested VPA concentrations had no effect (data not shown).
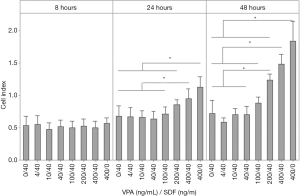
Third, CMSC29 were exposed to VPA and allowed to migrate toward a SDF-1α gradient. If VPA increased CXCR4, then CMSC29 migration towards SDF-1α would theoretically increase as well. Wells of the lower chamber were filled with α-MEM medium containing 40 ng/mL SDF-1α, while CMSC29 cells suspended in α-MEM medium containing 4, 10, 40, 100, 200 or 400 ng/mL VPA were added to duplicate wells of the upper chamber. Two controls were used for this experiment; cells that were not treated with VPA but with SDF-1α in the lower chamber, and cells that were treated with VPA without added SDF-1α in the lower chamber. Statistical analysis demonstrated a significant increase in the migration of CMSC29 treated with 400 ng/mL VPA after 24 hours. After 48 hours, there was a significant increase in the migration of CMSC29 treated with 200 and 400 ng/mL VPA (Figure 5). However, a significant increase in cell migration was also seen with VPA in the absence of SDF-1α (Figure 5). This indicates that VPA increases CMSC29 migration in a CXCR4 and SDF-1α independent manner.
Effect of VPA treatment on CMSC29 migration towards serum free medium
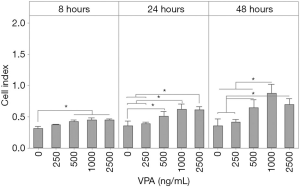
To confirm the effect of VPA treatment on CMSC29 cell migration, cells were suspended in non-supplemented α-MEM medium containing 250, 500, 1,000 or 2,500 ng/mL VPA, higher concentrations were used in this set of experiments to examine if the increase in migration was dose dependent. For each tested concentration, cells were added to duplicate wells of the upper chamber, with wells of the lower chamber filled with non-supplemented α-MEM medium. Statistical analysis showed that after 8, 24 and 48 hours, there was a significant increase in the migration of CMSC29 treated with 500, 1,000 and 2,500 ng/mL VPA compared with the control wells that had untreated cells (Figure 6).
Discussion
CMSC29 was created and characterized in this laboratory (1). Using a semi-quantitative scratch migration assay, CMSC29 cells migrated efficiently compared to primary CMSC in the absence of stimuli (1). In this study, the migration of CMSC29 cells was investigated with a well-established real-time, quantitative functional assay. Two cytokines (SDF-1α and HGF) and a pharmacological agent (VPA) were previously reported to stimulate MSC migration and were therefore screened for their ability to stimulate CMSC29 cell migration. Initial investigations showed that CMSC29 cells responded to stimulation, as AmnioMAXTM medium with added AmnioMAXTM growth supplements significantly increased cell migration. Since the constituents of AmnioMAXTM complete medium (i.e., AmnioMAXTM medium with growth supplements containing undisclosed quantity of FCS, AmnioMAXTM Product Information sheet, Publication number MAN0007317) significantly increased CMSC29 migration, all stimulants subsequently tested in the current study were prepared in serum free α-MEM medium.
CMSC29 cells were confirmed to be migratory, and capable of increasing their migration in response to a stimulus. The next step was to investigate if CMSC29 cells recapitulated the migratory behavior of primary CMSCs. Numerous studies have shown that SDF-1α and HGF stimulate MSC migration (4-8), including primary CMSC migration (from which the CMSC29 cell line is derived) (10). However, in this study, both SDF-1α and HGF failed to induce CMSC29 cell migration. The chemotactic effect of SDF-1α on CMSC29 was investigated further, by examining the surface expression of its receptor, CXCR4, on the cells. Flow cytometric analysis revealed that the expression of CXCR4 on CMSC29 was very low. In a MSC migration study, it was reported that, as a histone deacetylase inhibitor, VPA increased the levels of CXCR4 in BMMSC, which in turn increased their migration towards a SDF-1α gradient (12). Indeed in this study, treating CMSC29 cells with VPA significantly increased their migration, but, toward serum free medium, without the need for SDF-1α as a stimulus. This suggests the mechanism by which VPA stimulates CMSC29 cell migration is not mediated by the CXCR4-SDF-1α pathway. Another MSC migration study suggested that VPA increased BMMSC migration by increasing their release of trophic factors (16). Thus, this alternate mechanism may explain how VPA stimulated CMSC29 cell migration. However, further investigation is required before the pathway of VPA action on CMSC29 cell migration is fully understood.
VPA has been used for decades as a mood stabilizer for neuropsychiatric disorders. Many mechanisms of action have been postulated for VPA to explain its varied clinical effects, implying that VPA functions through multiple pathways. Two reported neuropsychiatric actions of VPA have been also implicated in cell migration studies, in addition to histone deacetylase inhibition, which regulates the transcription and expression of cellular factors (17). Another action of VPA is blocking the voltage-gated sodium channel, which is a cluster of cell membrane proteins that form a Na+ permeable pore (18). Blockage of the voltage-gated Na+ channel is also reported to be involved in the cell migration process (19,20). Along with the multiple mechanisms of action, another property of VPA that could contribute to its strong induction of CMSC29 cell migration is that VPA is taken up by cells via a non-receptor based mechanism (21,22). About 60% of VPA uptake by cells occurs through a carrier-mediated process with the remainder taken up by passive diffusion (23,24). For example, for its neuropsychiatric therapeutic effect, VPA is taken up through the blood-brain barrier via a medium-chain fatty acid transporter (23). Whereas, in a study employing trophoblast cells as a model to study drug transfer from mother to fetus, VPA cellular uptake was through a monocarboxylic acid transporter, which is temperature dependent and enhanced under acidic pH (25,26).
Despite our incomplete understanding of the precise mechanism by which VPA is taken up and exerts its neuropsychiatric effects, VPA is the drug of choice for an expanding range of neuropsychiatric conditions. Moreover, VPA is being considered for non-central nervous system related diseases, due to its effectiveness and well-established safety profile (27). Thus, the use of VPA could also be further explored in cellular therapy with the goal of enhancing MSC migration and engraftment. Since most cells lose vital surface receptors when cultured in vitro (4,13-15), a non-receptor based pre-transplant technique that employs VPA treatment of MSC may enhance their clinical benefits. In addition to stimulating MSC migration, pre-treating hematopoietic stem/progenitor cells with VPA enhanced their long term (20 weeks) engraftment into the bone marrow of immunodeficient mice (28). Moreover, it was also reported that culturing amniotic fluid stem cells in medium containing VPA, reprogrammed the cells to become pluripotent, creating induced pluripotent stem cells (iPSC). The pluripotent phenotype of VPA-treated cells was stable over time, with no signs of tumorigenicity in vitro or in vivo (29). Again, these data, along with the data obtained in this study, suggest that treatment with VPA could be a novel strategy to increase the migration potential of CMSC29 cells, and other sub-types of MSCs, in future stem cell-based therapies.
Acknowledgements
We would like to acknowledge Melissa Duggan, Janet Stevenson and Anthony Borg for their excellent technical assistance throughout the study.
Funding: The work was supported in part by King Abdulla International Medical Research Center (Grant No. RC08/114). Batla Al-Sowayan was supported by a dual scholarship from the Ministry of National Guard Health Affairs Scholarship Program, Ministry of National Guard-Kingdom of Saudi Arabia and the King Abdullah Scholarship Program, Ministry of Higher Education-Kingdom of Saudi Arabia.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Human Research Ethics Committee of the Royal Women’s Hospital, Parkville, Australia (No. 05/07).
References
- Qin SQ, Kusuma GD, Al-Sowayan B, et al. Establishment and characterization of fetal and maternal mesenchymal stem/stromal cell lines from the human term placenta. Placenta 2016;39:134-46. [Crossref] [PubMed]
- Ji JF, He BP, Dheen ST, et al. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells 2004;22:415-27. [Crossref] [PubMed]
- Neuss S, Becher E, Woltje M, et al. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells 2004;22:405-14. [Crossref] [PubMed]
- Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 2004;104:2643-5. [Crossref] [PubMed]
- Ma J, Ge J, Zhang S, et al. Time course of myocardial stromal cell-derived factor 1 expression and beneficial effects of intravenously administered bone marrow stem cells in rats with experimental myocardial infarction. Basic Res Cardiol 2005;100:217-23. [Crossref] [PubMed]
- Kitaori T, Ito H, Schwarz EM, et al. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum 2009;60:813-23. [Crossref] [PubMed]
- Vogel S, Trapp T, Borger V, et al. Hepatocyte growth factor-mediated attraction of mesenchymal stem cells for apoptotic neuronal and cardiomyocytic cells. Cell Mol Life Sci 2010;67:295-303. [Crossref] [PubMed]
- Vogel S, Peters C, Etminan N, et al. Migration of mesenchymal stem cells towards glioblastoma cells depends on hepatocyte-growth factor and is enhanced by aminolaevulinic acid-mediated photodynamic treatment. Biochem Biophys Res Commun 2013;431:428-32. [Crossref] [PubMed]
- Vogel S, Chatterjee M, Metzger K, et al. Activated platelets interfere with recruitment of mesenchymal stem cells to apoptotic cardiac cells via high mobility group box 1/Toll-like receptor 4-mediated down-regulation of hepatocyte growth factor receptor MET. J Biol Chem 2014;289:11068-82. [Crossref] [PubMed]
- Abumaree MH, Al Jumah MA, Kalionis B, et al. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev 2013;9:16-31. [Crossref] [PubMed]
- Sztajnkrycer MD. Valproic acid toxicity: overview and management. J Toxicol Clin Toxicol 2002;40:789-801. [Crossref] [PubMed]
- Tsai LK, Leng Y, Wang Z, et al. The mood stabilizers valproic acid and lithium enhance mesenchymal stem cell migration via distinct mechanisms. Neuropsychopharmacology 2010;35:2225-37. [Crossref] [PubMed]
- Kollar K, Cook MM, Atkinson K, et al. Molecular mechanisms involved in mesenchymal stem cell migration to the site of acute myocardial infarction. Int J Cell Biol 2009;2009:904682. [Crossref] [PubMed]
- Pelekanos RA, Ting MJ, Sardesai VS, et al. Intracellular trafficking and endocytosis of CXCR4 in fetal mesenchymal stem/stromal cells. BMC Cell Biol 2014;15:15. [Crossref] [PubMed]
- Von Luttichau I, Notohamiprodjo M, Wechselberger A, et al. Human adult CD34- progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev 2005;14:329-36. [Crossref] [PubMed]
- Cho GW, Kang BY, Kim KS, et al. Effects of valproic acid on the expression of trophic factors in human bone marrow mesenchymal stromal cells. Neurosci Lett 2012;526:100-5. [Crossref] [PubMed]
- Chiu CT, Wang Z, Hunsberger JG, et al. Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev 2013;65:105-42. [Crossref] [PubMed]
- Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol 2003;4:207. [Crossref] [PubMed]
- Shaya D, Kreir M, Robbins RA, et al. Voltage-gated sodium channel (NaV) protein dissection creates a set of functional pore-only proteins. Proc Natl Acad Sci U S A 2011;108:12313-8. [Crossref] [PubMed]
- Schwab A, Fabian A, Hanley PJ, et al. Role of ion channels and transporters in cell migration. Physiol Rev 2012;92:1865-913. [Crossref] [PubMed]
- Manji HK, Bebchuk JM, Moore GJ, et al. Modulation of CNS signal transduction pathways and gene expression by mood-stabilizing agents: therapeutic implications. J Clin Psychiatry 1999;60 Suppl 2:27-39; discussion 40-1, 113-6.
- Gould TD, Chen G, Manji HK. Mood stabilizer psychopharmacology. Clin Neurosci Res 2002;2:193-212. [Crossref] [PubMed]
- Adkison KD, Shen DD. Uptake of valproic acid into rat brain is mediated by a medium-chain fatty acid transporter. J Pharmacol Exp Ther 1996;276:1189-200. [PubMed]
- Naora K, Ichikawa N, Nishimura N, et al. Saturable transport of valproic acid in rat choroid plexus in vitro. J Pharm Sci 1996;85:423-6. [Crossref] [PubMed]
- Utoguchi N, Watanabe Y, Takase Y, et al. Carrier-mediated absorption of salicylic acid from hamster cheek pouch mucosa. J Pharm Sci 1999;88:142-6. [Crossref] [PubMed]
- Ushigome F, Takanaga H, Matsuo H, et al. Uptake mechanism of valproic acid in human placental choriocarcinoma cell line (BeWo). Eur J Pharmacol 2001;417:169-76. [Crossref] [PubMed]
- Wang ZF, Fessler EB, Chuang DM. Beneficial effects of mood stabilizers lithium, valproate and lamotrigine in experimental stroke models. Acta Pharmacol Sin 2011;32:1433-45. [Crossref] [PubMed]
- Vulcano F, Milazzo L, Ciccarelli C, et al. Valproic acid affects the engraftment of TPO-expanded cord blood cells in NOD/SCID mice. Exp Cell Res 2012;318:400-7. [Crossref] [PubMed]
- Moschidou D, Mukherjee S, Blundell MP, et al. Valproic acid confers functional pluripotency to human amniotic fluid stem cells in a transgene-free approach. Mol Ther 2012;20:1953-67. [Crossref] [PubMed]
Cite this article as: Al-Sowayan B, Keogh RJ, Abumaree M, Georgiou HM, Kalionis B. Valproic acid stimulates in vitro migration of the placenta-derived mesenchymal stem/stromal cell line CMSC29. Stem Cell Investig 2019;6:3.


