Stem cell therapy for patients with diabetes: a systematic review and meta-analysis of metabolomics-based risks and benefits
Introduction
Diabetes mellitus (DM) is a major health problem and the leading cause of death in the world; it is particularly responsible for 4 million deaths per year (1). The prevalence and incidence of DM in many societies, especially in developing countries, have been increasing more rapidly (2). In 1985, pieces of evidences revealed that 30 million people worldwide had DM; moreover, up to 230 million individuals were affected by 2008; and by the year 2025, the number of individuals with DM is expected to reach 300 million (3).
The traditional therapeutic methods such as administration of exogenous insulin through daily injections is the most prominent treatment for DM, but its administration is frequently associated with failure in glucose metabolism control, which ultimately leads to hyperglycemia episodes. Stem cell therapy holds a promising strategy to avoid the issues related to insulin daily injections. It is expected that this therapeutic method should produce, store, and supply insulin to maintain glucose homeostasis. Cell-based therapies are aimed at producing functional insulin-secreting β-cells to completely cure diabetes (4-6).
Over the years, researchers have been searching for ways to replace the destroyed beta cells in the pancreas (insulin-producing cells) with healthy ones by the immune system of a person with DM. Nowadays, the latest method of transplantation and replacement of pancreatic cells, in which clusters of insulin-producing cells, called islet, are transplanted from a donor pancreas to another liver, have provided excellent results for the treatment of type 1 diabetes patients (7). Changing the amount of insulin is often accompanied by a drop in blood glucose level or hypoglycemia, which can lead to medical emergencies such as coma. On the contrary, in the islet transplantation technique if the transplant works properly, the results will be very promising; thus, glycemic control is done by the body itself, and the amount of insulin needed is given to the body with respect to the amount of body glucose in the body; and homeostasis is done normally. Stem cell therapy for DM, which is in fact an attempt to eliminate insulin injections, leads to complications such as infectious diseases and cancer (8-10). Side-effects can occur at any time during, immediately, or several days or months after stem cell transplantation. Short-term side-effects (acute) usually occur within the first 100 days post-transplantation, while long-term side-effects (chronic) usually occur after 100 days or more after transplantation. Infectious diseases are among the most common primary side effects of stem cell transplantation which occurs due to very low white blood cell count and weak immune system. Though bacterial infections are the most common, viral or fungal infections may also occur.
To ensure that the emerging field of stem cell therapy fulfills its promise to patients with diabetes, we must first understand its risks and benefits; thus, if there will be greater health benefits and fewer risks, it may lead to developing a novel therapeutic approach based on sound science. A systematic review and meta-analysis was performed using all available clinical trials to determine the benefits and risks associated with stem cell therapy in patients with diabetes (both T1DM and T2DM).
Methods
This systematic review and meta-analysis was conducted according to the guidelines of the Centre for Reviews and Dissemination for undertaking systematic reviews (11), as well as the Meta-analysis of Observational Studies in Epidemiology (MOOSE) (12), and PRISMA guidelines (13). This research was approved by Ethics Committee of Tehran University of Medical Sciences (TUMS).
Types of studies
All relevant clinical trials examining the effectiveness of stem cells for the treatment of patients with diabetes were included without restrictions on publication status.
Types of participants
There was no limitation on age and sex for the inclusion in the study. Participants were excluded if they had further pathologies or changed endocrine status other than diabetes.
Types of interventions
Stem cell therapy is a method of using various types of stem cell in the treatment of T1DM and T2DM. The interventions and comparison include: (I) stem cell therapy compared to the control group; (II) stem cell therapy compared to placebo; (III) stem cell therapy compared to traditional treatment.
Types of outcome measures
Outcome measures included the type of stem cells [e.g., bone marrow mesenchymal stem cells (BM-MSCs), BM hematopoietic stem cells (BM-HSCs), umbilical cord MSCs (UC-MSCs), adipose stem cells (ASCs), mesenchymal precursor cells (MPCs), fetal stem cells (FSCs)], number of injected cells, method of stem cell delivery (intravenous or intrapancreatic administration), and follow-up time after cell therapy.
Search strategy
All studies on stem cells therapy in the treatment of diabetes were entered with no time limitation (all studies conducted by the end of July, 2017). A literature search strategy was developed using medical subject headings (MeSH) and keywords.
Electronic bibliographic databases
The following electronic bibliographic databases were searched: PubMed, Scopus, Web of Science, EMBASE and the Cochrane Library (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL). The search strategy included stem cells, progenitor cells, BM, BM-HSCs, BM-MSCs, UC-MSCs, ASCs, MPCs, FSCs, DM, T1DM, T2DM, and hyperglycemia. Limitation was placed on the search using English language and human subject filters. The search strategy for PubMed is shown in Figure S1.
Searching other resources
To ensure literature saturation, reference lists of selected studies or relevant reviews were scanned.
Inclusion criteria
Articles fulfilling the following criteria, including clinical trial studies on various stem cell therapies for both T1DM and T2DM, were considered. Besides, no language limitation was applied.
Exclusion criteria
Other article types such as reviews (narrative or systematic), observational studies, commentaries, letters to the editor, case series or case reports, and pooled analyses of original data were excluded.
Data collection
Selection of studies
After an electronic search, the records were uploaded using EndNote software. Titles and abstracts of studies were retrieved using the search strategy and those from additional resources were reviewed individually by two authors (F Rahim and K Shirbandi) to detect studies that possibly meet the inclusion criteria. The full text of these possibly qualified articles was retrieved and evaluated individually by the same authors.
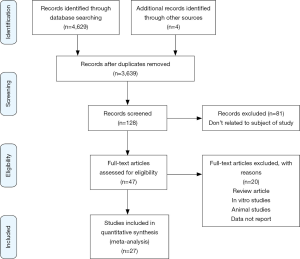
Any disagreements between the two authors were resolved through discussion. The whole process of study selection is summarized in the PRISMA flow diagram.
Data extraction and management
A standardized data collection form was used for assessing the quality of selected studies, as well as for evidence synthesis. The extracted information includes:
- General information (author, title, publication year, journal, location).
- Participant demographics and baseline characteristics [sample size, mean (range) age].
- Details of the intervention (cell type, number of cells injected, method of cell) delivery (intravenous or intra-pancreatic administration).
- Control conditions (no treatment, placebo therapy).
- Outcome measures (reported outcomes, adverse events, follow-up time and mechanism).
Assessment of risk of bias in included studies
The risk of bias was assessed independently by the two authors using the CONSORT checklist for reporting details of intervention for the treatment of diabetes (14).
The following domains were assessed for the risk of bias:
- Selection bias in terms of random sequence generation and allocation concealment.
- Performance bias in terms of blinding of participants, investigators and outcome assessors.
- Detection bias in terms of blinding of outcome assessment.
- Attrition bias in terms of incomplete outcome data.
- Reporting bias in terms of selective outcome reporting.
- Other bias in terms of for example, conflicts of interest, follow-up, non-intention-to-treat or per-protocol analysis. Disagreements between the authors in certain articles were resolved by discussion.
Measures of treatment effect
Review Manager 5.3 was used for data analysis and quantitative data synthesis. The treatment effect was measured using standardized mean difference (SMD) with 95% confidence intervals (CIs) in the case of continuous variables. Besides, a risk ratio (RR) with 95% CIs for analysis was used for categorical variables.
Unit of analysis issues
In the included trials, subjects were randomized into two intervention groups; and a measurement for each outcome was collected and analyzed. Data from the parallel group-designed and cross-over designed trials were included.
Dealing with missing data
Attempt was made to contact the corresponding authors of the studies that had missing or insufficient data. If it is not possible to get the missing data, then only the available data were analyzed and a sensitivity analysis was performed to determine whether the results are inconsistent.
Assessment of heterogeneity
Heterogeneity between the studies in terms of the measures of effect was assessed using both the Chi-square based Q test and I2 statistics. The result of the Q test was considered statistically significant at P<0.1. If the I2<50%, the study would be considered as not having heterogeneity. However, I2≥50% indicated significant statistic heterogeneity which would be reported accordingly, and meta-regression or a subgroup analysis was performed to explore the possible causes.
Assessment of reporting biases
Publication bias was assessed using the Egger test. Also, Funnel plots were used to detect possible reporting biases.
Data synthesis
Meta-analysis was performed using Review Manager 5.3. The results of Q test were presented using either a fixed effect or random effect models. In case of significant statistical heterogeneity, a random effect model was used; otherwise, a fixed effect model was used. Also, subgroup analyses or meta-regression were conducted if heterogeneity was present.
Subgroup analysis
If significant heterogeneity was observed, subgroup analyses were performed using both following assumptions:
- Comparison between stem cell therapy and no treatment.
- Comparison between stem cell therapy and placebo.
Sensitivity analysis
A sensitivity analysis was conducted considering the quality of selected studies (risk of bias) in order to investigate potential sources of heterogeneity. The methodological quality was assessed based on sample size and the effect of missing data. The meta-analysis was repeated and low-quality studies were excluded. The results were compared and discussed according to the extracted results from other studies.
Ethics and dissemination
In this study, ethical approval was not required because the data were extracted from peer-reviewed publications.
Ethical approval
This research was approved by the Ethics Committee of Tehran University of Medical Sciences, Tehran, Iran.
Results
Search results and description of studies
Overall, a total of 4,629 relevant studies were initially found. After removing 990 duplicates studies and the title-abstract screening also excluded 3,639 studies, a total of 27 studies were finally used for the systematic review and meta-analysis. Figure 1 outlines the search method and the number of studies identified and selected during each phase of the search.
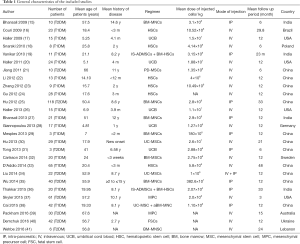
Table 1 provides a detailed overview of the 27 studies. Out of the 27 trials, the mean patient age with diabetes was 31.71±21.47 years. The total of the patients were 705 subjects among whom 245 were females (34.75%) and the remaining were male. Stem cell therapy had been practiced in patients with both T1DM (16 studies, 342 patients) and T2DM (11 studies, 363 patients).
Full table
Given the source of cells, six trials used HSCs (149 patients), six studies used BM-mononuclear cells (BM-MNCs) (195 patients), five studies used UCB (74 patients), two trials used UC-MSCs (51 patients), 3 studies used a combination of various stem cells (73 patients), one study used BM-MSCs (20 patients), and one study used placenta-derived MSCs (PD-MSCs, 10 patients). Two studies used MPCs (91 patients) and one study used FSCs (42 patients). These studies locations were India (n=4), Brazil (n=1), USA (n=4), Poland (n=1), China (n=12), Germany (n=1), Sweden (n=1), Australia (n=1), Ukraine (n=1), and Lebanon (n=1).
The outcome of stem cell therapy for T1DM
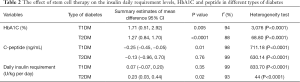
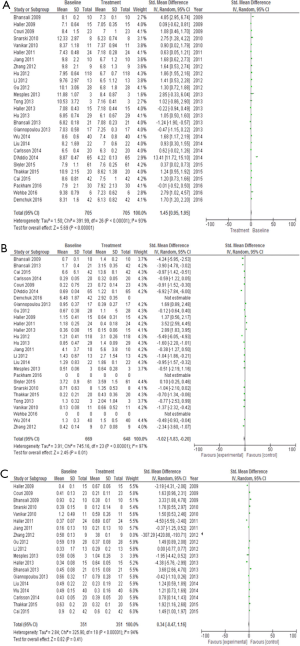
The mean age of patients with T1DM was 17.31±11.08 years. Stem cell therapy was performed in 16 trials [342 patients with T1DM, 132 females (38.59%)] (Table 1). Stem cell therapy improved the c-peptide levels as well as glycosylated hemoglobin (HbA1C), and had a positive effect on these variables, but induced a negative impact on insulin daily requirement and failed to resolve this problem (Table 2, Figures 2 and 3).
Full table
The outcome of stem cell therapy for T2DM
The mean patient age with T2DM was 56.20±7.49 years. Stem cell therapy was performed in 11 studies [363 patients with T2DM, 113 females (31.12%)] (Table 1). Stem cell therapy improved the insulin daily requirement levels, as well as HbA1C, and had a positive effect on these variables, but had a negative impact on c-peptide (Table 2, Figures 2 and 3).
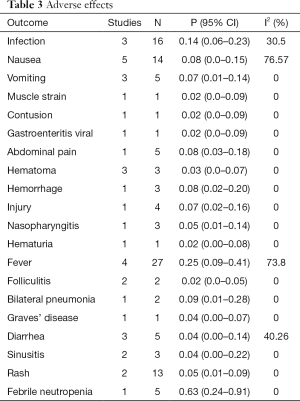
Our review concluded that 20 various adverse events were reported for patients treated with stem cells among which fever was the highest reported symptom with an incidence rate of 0.14%. Also, muscle strain, contusion, viral gastroenteritis, hematuria, and folliculitis complications were the lowest reported adverse effects, with an incidence rate of 0.02% (Table 3).
Full table
Discussion
With the increasing incidence of DM, several approaches to clinically deal with this disease have been reviewed so far. One of these new methods is the application of stem cell therapy to improve diabetic complications, especially in T1DM. With the popularity of cell therapy, in addition to increasing longevity, a healthier life is promising for humans (42,43).
To the best of our knowledge, the present meta-analysis considers all available evidence on stem cell therapy for DM and critically assesses and quantifies the safety and efficacy of this approach as well. All types of stem cell therapies applied in both T1DM and T2DM patients were included. Our analysis showed that the intravenous administration of CD34+ BM-HSC is a better treatment approach for T1DM than others. Nevertheless, a dearth of clinical studies conducted in this field is apparent (19,36). Although MSCs are widely reported to yield the greatest therapeutic success (19,21,30,32,34,36), FSCs, exclusively used for T2DM treatment, lead to decrease in hyperinsulinemia and consequently lower insulin resistance (40). Recently, the safety, tolerability, and therapeutic effects of adult allogeneic bone-marrow derived MPCs in patients with and without moderate to severe diabetic nephropathy have been explored and it demonstrated that the safety of rexlemestrocel-L in diabetic nephropathy with suggestive effects on renal function needs to be confirmed in appropriately larger trials (37,39). But, UCB failed to improve C-peptide, HbA1c, and insulin utilization levels in T1DM patients (17,20,26,28,31). Thus, stem cell therapy seems to be a safer form of transplantation therapy for the treatment of DM compared to whole organ and islet transplantation.
Limitation
There were some markers to evaluate DM in patients. But some of the studies reported c-peptide, HbA1C and insulin daily requirement for this, and a few studies reported another marker which is not enough for meta-analysis.
Conclusions
Stem cells have the ability to be self-renewed and differentiate into a variety of cells, including blood, heart, nervous and cartilage cells. Paradoxically, it has been stated that these cells also have the potential to form cancer cells. These possible risks necessitate that both medical specialists and patients should proceed the treatment with caution; thus, it is critically crucial to conduct further research on stem cell therapy considering their risk and benefits in the first place.
Acknowledgements
None.
Footnote
Conflicts of Interest: This research was the result of a collaborative research between Deputy of Research Affairs, Ahvaz Jundishapur University of Medical Sciences, Ahvaz-Iran and Metabolomics and Genomics Research Center, of Endocrinology and Metabolism Molecular-Cellular Sciences Institute affiliated to Tehran University of medical sciences, Tehran, Iran. The abstract of this paper was presented at the International Conference on Diabetes & Endocrinology Disorders May 15-17, 2017 Dubai, UAE as an oral presentation/conference talk with interim findings.
Ethical Statement: This research was approved by Ethics Committee of Tehran University of Medical Sciences (TUMS).
References
- Tabish SA. Is Diabetes Becoming the Biggest Epidemic of the Twenty-first Century? Int J Health Sci (Qassim) 2007;1:V-VIII. [PubMed]
- Ramachandran A, Snehalatha C, Shetty AS, et al. Trends in prevalence of diabetes in Asian countries. World J Diabetes 2012;3:110. [Crossref] [PubMed]
- Scully T. Diabetes in numbers. Nature 2012;485:S2-3. [Crossref] [PubMed]
- Farooq T, Rehman K, Hameed A, et al. Stem Cell Therapy and Type 1 Diabetes Mellitus: Treatment Strategies and Future Perspectives. Adv Exp Med Biol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Rattananinsruang P, Dechsukhum C, Leeanansaksiri W. Establishment of Insulin-Producing Cells From Human Embryonic Stem Cells Underhypoxic Condition for Cell Based Therapy. Front Cell Dev Biol 2018;6:49. [Crossref] [PubMed]
- Wu H, Mahato RI. Mesenchymal stem cell-based therapy for type 1 diabetes. Discov Med 2014;17:139-43. [PubMed]
- Gaba RC, Garcia-Roca R, Oberholzer J. Pancreatic islet cell transplantation: an update for interventional radiologists. J Vasc Interv Radiol 2012;23:583-94. [Crossref] [PubMed]
- Khamaisi M, Balanson SE. Stem Cells for Diabetes Complications: A Future Potential Cure. Rambam Maimonides Med J 2017;8:e0008. [Crossref] [PubMed]
- Yuza Y, Glatt KA, Jiang J, et al. Allele-dependent variation in the relative cellular potency of distinct EGFR inhibitors. Cancer Biol Ther 2007;6:661-7. [Crossref] [PubMed]
- Saki N, Jalalifar MA, Soleimani M, et al. Adverse effect of high glucose concentration on stem cell therapy. Int J Hematol Oncol Stem Cell Res 2013;7:34-40. [PubMed]
- Undertaking systematic reviews of research of effectiveness. CRD’s Guidance for Those Carrying Out or Commissioning Reviews. 2nd ed. York, UK: Centre for Reviews and Dissemination, 2001.
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]
- Panic N, Leoncini E, de Belvis G, et al. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One 2013;8:e83138. [Crossref] [PubMed]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [Crossref] [PubMed]
- Bhansali A, Upreti V, Khandelwal N, et al. Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev 2009;18:1407-16. [Crossref] [PubMed]
- Couri CE, Oliveira MC, Stracieri AB, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA 2009;301:1573-9. [Crossref] [PubMed]
- Haller MJ, Wasserfall CH, McGrail KM, et al. Autologous umbilical cord blood transfusion in very young children with type 1 diabetes. Diabetes Care 2009;32:2041-6. [Crossref] [PubMed]
- Snarski E, Milczarczyk A, Torosian T, et al. Independence of exogenous insulin following immunoablation and stem cell reconstitution in newly diagnosed diabetes type I. Bone Marrow Transplant 2011;46:562-6. [Crossref] [PubMed]
- Vanikar A, Dave S, Thakkar U, et al. Cotransplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: a novel therapy for insulin-dependent diabetes mellitus. Stem Cells Int 2010;2010.
- Haller MJ, Wasserfall CH, Hulme MA, et al. Autologous umbilical cord blood transfusion in young children with type 1 diabetes fails to preserve C-peptide. Diabetes Care 2011;34:2567-9. [Crossref] [PubMed]
- Jiang R, Han Z, Zhuo G, et al. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Frontiers of medicine 2011;5:94-100. [Crossref] [PubMed]
- Li L, Shen S, Ouyang J, et al. Autologous hematopoietic stem cell transplantation modulates immunocompetent cells and improves β-cell function in Chinese patients with new onset of type 1 diabetes. J Clin Endocrinol Metab 2012;97:1729-36. [Crossref] [PubMed]
- Zhang X, Ye L, Hu J, et al. Acute response of peripheral blood cell to autologous hematopoietic stem cell transplantation in type 1 diabetic patient. PLoS One 2012;7:e31887. [Crossref] [PubMed]
- Gu W, Hu J, Wang W, et al. Diabetic ketoacidosis at diagnosis influences complete remission after treatment with hematopoietic stem cell transplantation in adolescents with type 1 diabetes. Diabetes Care 2012;35:1413-9. [Crossref] [PubMed]
- Hu J, Li C, Wang L, et al. Long term effects of the implantation of autologous bone marrow mononuclear cells for type 2 diabetes mellitus. Endocr J 2012;59:1031-9. [Crossref] [PubMed]
- Haller MJ, Wasserfall CH, Hulme MA, et al. Autologous umbilical cord blood infusion followed by oral docosahexaenoic acid and vitamin D supplementation for C-peptide preservation in children with Type 1 diabetes. Biol Blood Marrow Transplant 2013;19:1126-9. [Crossref] [PubMed]
- Bhansali A, Asokumar P, Walia R, et al. Efficacy and safety of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus: a randomized placebo-controlled study. Cell Transplant 2014;23:1075-85. [Crossref] [PubMed]
- Giannopoulou EZ, Puff R, Beyerlein A, et al. Effect of a single autologous cord blood infusion on beta-cell and immune function in children with new onset type 1 diabetes: a non-randomized, controlled trial. Pediatr Diabetes 2014;15:100-9. [Crossref] [PubMed]
- Mesples A, Majeed N, Zhang Y, et al. Early immunotherapy using autologous adult stem cells reversed the effect of anti-pancreatic islets in recently diagnosed type 1 diabetes mellitus: Preliminary results. Med Sci Monit 2013;19:852-7. [Crossref] [PubMed]
- Hu J, Yu X, Wang Z, et al. Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J 2013;60:347-57. [Crossref] [PubMed]
- Tong Q, Duan L, Xu Z, et al. Improved insulin secretion following intrapancreatic UCB transplantation in patients with T2DM. J Clin Endocrinol Metab 2013;98:E1501-4. [Crossref] [PubMed]
- Carlsson PO, Schwarcz E, Korsgren O, et al. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 2015;64:587-92. [Crossref] [PubMed]
- D’Addio F, Vasquez AV, Nasr MB, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in new-onset type 1 diabetes: a multicenter analysis. Diabetes 2014;63:3041-6. [Crossref] [PubMed]
- Liu X, Zheng P, Wang X, et al. A preliminary evaluation of efficacy and safety of Wharton's jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Res Ther 2014;5:57. [Crossref] [PubMed]
- Wu Z, Cai J, Chen J, et al. Autologous bone marrow mononuclear cell infusion and hyperbaric oxygen therapy in type 2 diabetes mellitus: an open-label, randomized controlled clinical trial. Cytotherapy 2014;16:258-65. [Crossref] [PubMed]
- Thakkar UG, Trivedi HL, Vanikar AV, et al. Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy 2015;17:940-7. [Crossref] [PubMed]
- Skyler JS, Fonseca VA, Segal KR, et al. Allogeneic Mesenchymal Precursor Cells in Type 2 Diabetes: A Randomized, Placebo-Controlled, Dose-Escalation Safety and Tolerability Pilot Study. Diabetes Care 2015;38:1742-9. [Crossref] [PubMed]
- Cai J, Wu Z, Xu X, et al. Umbilical Cord Mesenchymal Stromal Cell With Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. Diabetes Care 2016;39:149-57. [Crossref] [PubMed]
- Packham DK, Fraser IR, Kerr PG, et al. Allogeneic Mesenchymal Precursor Cells (MPC) in Diabetic Nephropathy: A Randomized, Placebo-controlled, Dose Escalation Study. EBioMedicine 2016;12:263-9. [Crossref] [PubMed]
- Demchuk M, Ivankova O, Klunnyk M, et al. Efficacy of Fetal Stem Cells use in Complex Treatment of Patients with Insulin-resistant Type 2 Diabetes Mellitus. J Stem Cell Res Ther 2016;6:342. [Crossref]
- Wehbe T, Chahine NA, Sissi S, et al. Bone marrow derived stem cell therapy for type 2 diabetes mellitus. Stem Cell Invest 2016;3.
- Douay L, Lapillonne H, Turhan AG. Stem cells--a source of adult red blood cells for transfusion purposes: present and future. Crit Care Clin 2009;25:383-98. Table of Contents. [Crossref] [PubMed]
- Corsten MF, Shah K. Therapeutic stem-cells for cancer treatment: hopes and hurdles in tactical warfare. Lancet Oncol 2008;9:376-84. [Crossref] [PubMed]
Cite this article as: Rahim F, Arjmand B, Shirbandi K, Payab M, Larijani B. Stem cell therapy for patients with diabetes: a systematic review and meta-analysis of metabolomics-based risks and benefits. Stem Cell Investig 2018;5:40.