Breast cancer stem cells and the challenges of eradication: a review of novel therapies
Prevalence of breast cancer
Breast cancer accounts for 30% of all cancers diagnosed in women with greater than 1,677,000 new cases and over 520,000 deaths per year worldwide (1). Despite recent advancements in detection and treatment, mortality of the disease is expected to increase 20% by the year 2020 with >95% of new cases occurring in women older than 40 years of age (2,3). Approximately 40% of patients who are initially diagnosed with non-invasive breast cancer progress to malignancy and experience disease recurrence despite undergoing treatments such as chemotherapy and/or adjuvant care. Furthermore, 70% of these cases experience a metastatic relapse within 5 years (4). Due to the heterogeneous nature of this disease, the effectiveness of recent therapies has been limited (5).
In this review, we provide a clinical discussion of metastatic breast cancer including a review of the breast cancer stem cell (CSC), its signaling pathways and immunological/pathological markers, and novel therapies designed for targeted treatment.
Types of breast cancer
Clinical character
Breast cancer is a molecularly, pathologically, and epidemiologically heterogeneous disease. Clinically, invasive breast cancers can be classified into three groups: early breast cancer (stages I, IIa, and IIb), locally advanced breast cancer (stages IIIa, IIIb, and IIIc), and advanced breast cancer (stage IV) presenting with distant metastases beyond the regional lymph nodes (6,7).
Immunological subtype
Markers in breast cancer have long been appreciated by researchers to define and identify options for targeted treatment. There are four immunological subtypes of breast cancer containing a combination of the three chief markers; estrogen receptor (ER), progesterone receptor (PR), and human epidermal receptor 2 (HER2) which are routinely defined in the clinic in order to optimize patient outcomes (8).
Gene expression profile
Global gene expression analyses have given us a closer look at this complex heterogeneous disease. Six intrinsic molecular subtypes (luminal A, luminal B, basal-like, HER2-overexpressing, Claudin-low, and normal breast-like) of breast cancer have been identified and have provided a deeper understanding of the differences in gene expression between tumors presenting with these variable immunological markers (9,10). For example, there is clear evidence that “basal-like” and “luminal” subgroups differ with respect to outcome of disease in patients with locally advanced breast cancer (11) (Figure 1).
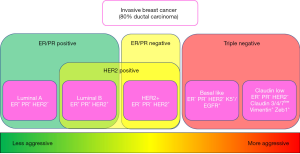
CSCs
Cancers are believed to arise from a series of genetic mutations that occur as a result of cellular instability and/or oncogene-induced plasticity (12). First discovered in acute myeloid leukemia (AML), CSCs have played a major role in the advancement of cancer research (13). CSCs have led to a remodeling of our cancer hypothesis and have been the subject of concentrated research as potential targets for cancer therapies (14-18). The majority of cells within solid tumors are more differentiated and have limited self-renewal abilities (19). CSCs, on the other hand, make up a small subpopulation of cells within a tumor and are responsible for tumor, initiation, formation and recurrence (14). CSCs have been shown to undergo symmetric self-renewal giving rise to two identical pluripotent daughter CSCs, as well as, an asymmetric division producing a more differentiated tumor progenitor cell (TPC) and an identical daughter CSC. This self-promoting mechanism results in an increased number of CSCs as the tumor grows as well as expansion of the overall tumor in size (20). Furthermore, CSCs have been shown to undergo epithelial-to-mesenchymal-transition (EMT), a known mechanism in metastasis (21) (Figure 2).
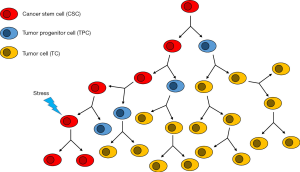
Breast CSCs (BCSCs)
BCSCs are derived from human breast tumors with a series of markers, including CD44, CD24, CD133, epithelial cell adhesion molecule (EpCAM), nestin, ganglioside GD2, CD49f, CD61, CXCR4, CXCL1, HMGCS, CD166, CD47, ALDH1, and ABCG2 (22-25). However, cell markers CD44+/CD24− have been established as minimum surface markers for BCSCs (14). CD44 is a transmembrane glycoprotein that binds to many extracellular matrix proteins, of which hyaluronic acid is the most common. Hyaluronic acid is a key component outside the cell that aids in the control and regulation of cell adhesion, migration, and invasive proliferation. Further, the interaction between hyaluronic acid and osteopontin is believed to lead to tumor progression (26,27). High levels of CD44 mRNA and protein expression levels in breast cancer has been linked to significantly worse overall survival (28). Additionally, elevated levels of CD44 expression was found in tumor-forming cells in numerous cancers (29). Clearly, CD44 is believed to be a valid biomarker for CSCs (30). The absence of CD24, another extracellular glycoprotein, has been shown to increase tumor growth and promote metastasis (31). CD133 has been used in combination with the CD44+/CD24− phenotype to isolate BCSCs (32). Interestingly, expression of aldehyde dehydrogenase 1 (ALDH1), an intracellular enzyme that oxidizes aldehydes and retinol, is considered one of the top markers for CSCs in the breast and has been shown to illicit remarkable treatment resistance, a more aggressive phenotype, and ultimately poorer outcomes in patients (33). Despite differences among different subtypes of breast cancer, positive ALDH1 expression has been shown in significantly large proportions compared to other CSC-related markers (34). Although numerous studies have contributed to a better understanding of BCSC surface markers, the picture is still not fully understood. It is often observed that CSCs do not express the same surface markers, or that these markers are not exclusive to CSC and are also variably expressed in cells throughout the breast and body. As a consequence, isolation of BCSCs has been challenging. Currently, there exist no standardized criteria in place to identify BCSCs in human breast cancer (35).
BCSC signaling
Signaling pathways are essential for the regulation of normal stem cells. Many of these pathways are deregulated in CSCs which induce tumor formation. Most notable among these pathways are the Notch, Wnt/Frizzled/β-catenin, Hippo, and Hedgehog signaling cascades which are responsible for the formation of CSCs (36-38).
Notch signaling plays an essential role in normal stem cell maintenance and differentiation. Dysfunction of the Notch pathway has been linked to the development of breast cancer and is believed to be upregulated in a variety of cancers (39-42). The Notch pathways are composed of transmembrane receptors (Notch 1–4) which undergo cleavage, nuclear translocation, and subsequent gene activation upon binding Notch ligands. Notch activation via a constitutively active Notch receptor in normal epithelial cells has been shown to induce hyper-proliferation and breast tumor formation (43,44). Therapeutic resistance in breast CSCs is also believed to be associated with Notch signaling and has been an area of strong interest in cancer research (45).
Hippo signaling is a well-established in tissue homeostasis and tumorigenesis. Hippo signaling is modulated via two pairs of kinases, Mst1/2 and Lats1/2. Upon phosphorylation of downstream Yes-associated protein 1 (YAP1) or Lats1/2-induced TAZ transcription is inactivated and leads to cellular degradation, whereas, dephosphorylation leads to YAP/TAZ nuclear translocation and subsequent activation of transcription (46). Abnormal regulation of Hippo pathway leading to overexpression of YAP1 or TAZ has been shown to be elevated in numerous types of cancers and can directly promote tumorigenesis in mouse models (47). Further, metastatic breast tumors have been associated with BCSCs which express a remarkable TAZ abundance further suggesting the significance of YAP/TAZ in CSCs (48,49).
The Wnt/Frizzled/β-catenin pathway is an important regulator of normal breast development as well as abnormal tumorigenesis. The Wnt signaling proteins play an important role alongside the Frizzled family of cell surface receptors and the Dishevelled family of phosphoproteins to regulate the proteolytic degradation of β-catenin. β-catenin plays an unequivocal role in gene transcription that is involved in determining cell migration, cytoskeletal activity, cell polarity, and cellular differentiation and the inhibition of β-catenin signaling has been shown to prevent mammary development and cellular proliferation during pregnancy (50,51). Most notably, overexpression of Wnt signaling pathways led to breast tumor formation in transgenic mice and an increased number of progenitor cells in precancerous mammary glands (52,53).
Hedgehog signaling is another critical regulator of cell proliferation, stem cell maintenance, and cell fate, including cell self-renewal (54). The pathway is essential for the proper development of mammary epithelium and its disruption has been linked to human breast cancer (55). Previous studies have illustrated the interaction of hedgehog ligand with the patched (Ptch) receptor of a neighboring cell leading to the release of activated Gli which undergoes nuclear translocation to regulate gene expression. Gli-1 and Ptch-1 illicit regulatory negative feedback on hedgehog signaling which has been observed to be reduced or lost in a significant proportion of breast cancers (56-58). Further, components of hedgehog signaling have been correlated with activation of breast CSCs and high expression levels have also been linked to maintenance of the tumor microenvironment which results in autocrine activation of stroma via endogenous generation of Hedgehog ligands (59,60). Aberrant activation of the Hedgehog effector Gli-1 is linked to increased tumor formation and the development of breast cancers in experimental models (61).
Therapy resistance
It is well established that CSCs utilize multiple lines of self-defense against chemotherapeutic drugs and ionization therapies (62). Despite intensive studies in the past, the mechanisms by which breast tumors become chemoresistant is not fully understood (63). Tumor heterogeneity is a key product of CSCs and a key feature of therapy resistance, especially when specifically targeting CSC surface markers (64).
An overwhelming amount of chemotherapy drugs target cells undergoing proliferation. CSCs are predominantly in a resting G0 phase of the cell cycle. Thus, CSCs are inherently immune to the actions of drugs which rely on an overactive cell cycle (64). Further, CSCs under attack by radiation or chemotoxic agents upregulate IGF (insulin-like growth factor) type 1 receptor and increase secretion of IGF1. In the resting G0 phase, this expression pattern inhibits PI3K-AKT signaling and activates Fox03a slowing the cell cycle and stimulating self-renewal (65).
CSCs utilize ALDH1, a member of the NADP+ dependent super family of enzymes known for the physiological and detoxification mechanism involved in CSCs self-defense. ALDH1 functions by catalyzing the conversion of aldehyde to carboxylic acids, which accumulates as a result of chemotherapy, radiation, or other sources of oxidative stress (66). Downregulating ALDH1, by retinoic acid, has been shown to be an effective treatment in some cancers and a promising treatment sensitizing agent in solid mass breast tumors (67).
ABC transporter activation of ATP-dependent chemotoxin efflux is another mechanism CSCs employ to establish resistance against chemotherapeutic agents and other molecularly targeted therapies (68). Thus, targeting ABC transporters poses a potential mechanism to re-sensitize CSCs and inhibit this pathway of therapy resistance. However, ABC transporters play an important role in normal tissue physiology and their inhibition could lead to severe side effects (67).
Current approaches
Eradicating breast cancer is only possible if we overcome the challenges of effectively and specifically targeting breast CSCs. Despite their abundance, the majority of CSC markers are inadequate for targeting as they are also expressed on normal stem cells. CD44 is the most common CSC marker and is a major contributor to stemness (69). Despite numerous challenges associated with CD44 splicing and post-translational modification, anti-CD44 antibodies have been effective at inducing terminal differentiation of CSC resulting in reduced tumor growth and a significant decrease in metastasis (70,71).
Another key CSC marker is CD133 and treatment with a cytotoxic anti-CD133 antibody has proven effective at eradicating numerous cancers in vivo (72). Despite the effectiveness of this approach, targeting CD133 is a rather controversial strategy as its function in normal tissues is not yet fully understood. However, bi-specific antibodies have recently been developed to initiate a T cell response against CD133 (73).
Targeting CSC signaling pathways that play critical roles in self-renewal and defense has been an area of increasing research and clinical trials (74). The Notch pathway has been implicated particularly in breast CSCs and is thought to increase the rate of epithelial-mesenchymal transition ultimately contributing to an increase in metastasis of the CSCs (75). Numerous studies have shown inhibition of Notch signaling to resensitize BCSCs to chemotherapeutic agents and radiation therapy (76). In particular, Psoralidin, a plant-based inhibitor of Notch signaling has been shown to effectively decrease bulk tumor size, upregulate pro-apoptotic genes, and inhibit CSC proliferation and self-renewal (77).
Mediation of the Hippo signaling protein YAP/TAZ has been implicated as an important regulator and inhibitor of self-renewal in BCSCs (49). Overexpression of TAZ promoted tumor growth and an increase in the CSC phenotype, whereas, TAZ knockdown models reported a decrease in overall tumor size and significant decrease in CSC proliferation (78). These findings have indicated YAP/TAZ as an important target for the development of cancer therapies.
Dysregulation of the Hedgehog signaling pathway is believed to play a critical role in the formation of CSCs. Cyclopamine, a well-known Hedgehog antagonist used heavily to study tumor behavior, has been shown to deplete CSC populations via inhibition of CSC proliferation, and ultimately result in a decrease of the overall tumor size in multiple cancers (79-82). Currently, the most direct and potent inhibitor of SMO, a Hedgehog ligand, is vismodegib (83). However, its efficacy in treating breast cancer is not yet clear.
Hedgehog abnormalities are linked to dysfunction of the Wnt pathway which plays a role in maintaining the self-renewal capabilities of CSCs. Wnt/Frizzled/β-catenin inhibitors include non-steroidal anti-inflammatories (NSAIDs), COX-2 inhibitors, and glitazone anti-diabetic agents which have all shown promise pre-clinically as therapy agents capable of reducing the ability of CSC to self-renew (83,84). Additionally, anti-Frizzled receptor antibodies have proven effective at reducing tumor growth and regressing CSC populations (85). However, their use is not believed to be safe considering the importance of the Wnt pathway in normal tissue homeostasis (86).
In addition to targeting CSC surface markers, transporters, and signaling pathways, many studies have demonstrated decreased tumor growth by targeting the tumor microenvironment resulting in an increase in the effectiveness of chemotherapy (87,88). In particular, repertaxin, a non-competitive inhibitor of IL-8 cytokine is one example of such a drug proven to effectively target human BCSCs (89). Lastly, recent studies have demonstrated the use of cannabinoid receptor agonist, ACEA, as an effective agent to decrease the invasiveness of BCSCs (90).
Conclusions
There is compelling evidence that cancer is a disease manifested and maintained by stem cells. Breast cancer remains a major cause of morbidity and mortality in women worldwide. While tremendous amounts of research have been done to understand breast cancer, there is still much we do not fully understand. We have learned that CSCs are responsible for tumor initiation, development, metastasis, and most importantly recurrence after treatment. We have attempted to provide a representative overview of breast cancer prevalence, the stem of its manifestation, and novel therapies currently being explored to treat patients with this relentless disease.
Acknowledgements
This work was supported by the Tulane University School of Medicine DeBakey Scholars Program, the Dr. Howard and Brenda B. Sheridan Endowed Scholarship, the Frank P. Tagliarini MD Endowed Fund, and the William Strange Memorial Scholarship.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- GLOBOCAN Cancer Fact Sheet 2012, International Agency for Research on Cancer. Available online: http://globocan.iarc.fr/factsheets/cancers/breast.asp. Accessed: October 13, 2012.
- de Rinaldis E, Tutt A, Dontu G. Breast Cancer. Breast Pathol 2011:352-9.
- Gupta PB, Onder TT, Jiang G, et al. Identification of Selective Inhibitors of Cancer Stem Cells by High-Throughput Screening. Cell 2009;138:645-59. [Crossref] [PubMed]
- Stockler M, Wilcken NRC, Ghersi D, et al. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer. Cancer Treat Rev 2000;26:151-68. [Crossref] [PubMed]
- Hammer C, Fanning A, Crowe J. Overview of breast cancer staging and surgical treatment options. Cleve Clin J Med 2008;75:S10-6. [Crossref] [PubMed]
- Senkus E, Kyriakides S, Ohno S, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26 Suppl 5:v8-30. [Crossref] [PubMed]
- Bertos NR, Park M. Breast cancer—one term, many entities? J Clin Invest 2011;121:3789-96. [Crossref] [PubMed]
- Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol 2011;5:5-23. [Crossref] [PubMed]
- Parker JS, Mullins M, Cheang MC, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160-7. [Crossref] [PubMed]
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74. [Crossref] [PubMed]
- Aubele M, Werner M. Heterogeneity in breast cancer and the problem of relevance of findings. Anal Cell Pathol 1999;19:53-8. [Crossref] [PubMed]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730-7. [Crossref] [PubMed]
- Al-Hajj M, Wicha MS, Benito-Hernandez A, et al. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100:3983-8. [Crossref] [PubMed]
- Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003;63:5821-8. [PubMed]
- Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823-35. [Crossref] [PubMed]
- Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat Rev Cancer 2013;13:727-38. [Crossref] [PubMed]
- Chen K, Huang Y, Chen J. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin 2013;34:732-40. [Crossref] [PubMed]
- Al-Hajj M, Clarke MF. Self-renewal and solid tumor stem cells. Oncogene 2004;23:7274-82. [Crossref] [PubMed]
- Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res 2006;66:9339-44. [Crossref] [PubMed]
- Alison MR, Lin WR, Lim SM, et al. Cancer stem cells: in the line of fire. Cancer Treat Rev 2012;38:589-98. [Crossref] [PubMed]
- Collina F, Di Bonito M, Li Bergolis V, et al. Prognostic Value of Cancer Stem Cells Markers in Triple-Negative Breast Cancer. Biomed Res Int 2015;2015. [Crossref] [PubMed]
- Lee WJ, Kim SC, Yoon JH, et al. Meta-Analysis of Tumor Stem-Like Breast Cancer Cells Using Gene Set and Network Analysis. PLoS One 2016;11. [Crossref] [PubMed]
- Vaillant F, Asselin-Labat ML, Shackleton M, et al. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res 2008;68:7711-7. [Crossref] [PubMed]
- Cariati M, Naderi A, Brown JP, et al. Alpha-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. Int J Cancer 2008;122:298-304. [Crossref] [PubMed]
- Herrera-Gayol A, Jothy S. Adhesion proteins in the biology of breast cancer: contribution of CD44. Exp Mol Pathol 1999;66:149-56. [Crossref] [PubMed]
- Rangaswami H, Bulbule A, Kundu GC. Osteopontin: Role in cell signaling and cancer progression. Trends Cell Biol 2006;16:79-87. [Crossref] [PubMed]
- Xu H, Tian Y, Yuan X, et al. Enrichment of CD44 in basal-type breast cancer correlates with EMT, cancer stem cell gene profile, and prognosis. Onco Targets Ther 2016;9:431-44. [PubMed]
- Zöller M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer 2011;11:254-67. [Crossref] [PubMed]
- Basakran NS. CD44 as a potential diagnostic tumor marker. Saudi Med J 2015;36:273-9. [Crossref] [PubMed]
- Schabath H. CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. J Cell Sci 2006;119:314-25. [Crossref] [PubMed]
- Lawson JC, Blatch GL, Edkins AL. Cancer stem cells in breast cancer and metastasis. Breast Cancer Res Treat 2009;118:241-54. [Crossref] [PubMed]
- Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007;1:555-67. [Crossref] [PubMed]
- Bi X, Wu C, Han M, et al. Correlations of ALDH1 expression with molecular subtypes and ABCG2 in breast cancer. Gland Surg 2012;1:12-9. [PubMed]
- Lin CY, Barry-Holson KQ, Allison KH. Breast cancer stem cells: are we ready to go from bench to bedside? Histopathology 2016;68:119-37. [Crossref] [PubMed]
- Maugeri-Saccà M, Zeuner A, De Maria R. Therapeutic targeting of cancer stem cells. Front Oncol 2011;1:10. [Crossref] [PubMed]
- Muller JM, Chevrier L, Cochaud S, et al. Hedgehog, Notch and Wnt developmental pathways as targets for anti-cancer drugs. Drug Discov Today Dis Mech 2007;4:285-91. [Crossref]
- Merchant AA, Matsui W. Targeting Hedgehog - A cancer stem cell pathway. Clin Cancer Res 2010;16:3130-40. [Crossref] [PubMed]
- Harrison H, Farnie G, Howell SJ, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res 2010;70:709-18. [Crossref] [PubMed]
- Qiao L, Wong BC. Role of Notch signaling in colorectal cancer. Carcinogenesis 2009;30:1979-86. [Crossref] [PubMed]
- Pannuti A, Foreman K, Rizzo P, et al. Targeting Notch to Target Cancer Stem Cells. Clin Cancer Res 2010;16:3141-52. [Crossref] [PubMed]
- Wang Z, Ahmad A, Li Y, et al. Targeting notch to eradicate pancreatic cancer stem cells for cancer therapy. Anticancer Res 2011;31:1105-13. [PubMed]
- Mazzone M, Selfors LM, Albeck J, et al. Dose-dependent induction of distinct phenotypic responses to Notch pathway activation in mammary epithelial cells. Proc Natl Acad Sci 2010;107:5012-7. [Crossref] [PubMed]
- Callahan R, Raafat A. Notch Signaling in Mammary Gland Tumorigenesis. J Mammary Gland Biol Neoplasia 2001;6:23-36. [Crossref] [PubMed]
- Phillips TM, McBride WH, Pajonk F. The response of CD24-/low/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst 2006;98:1777-85. [Crossref] [PubMed]
- Stanger BZ. Quit your YAPing: a new target for cancer therapy. Genes Dev 2012;26:1263-7. [Crossref] [PubMed]
- Avruch J, Zhou D, Fitamant J, et al. Protein kinases of the Hippo pathway: Regulation and substrates. Semin Cell Dev Biol 2012;23:770-84. [Crossref] [PubMed]
- Chan SW, Lim CJ, Guo K, et al. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res 2008;68:2592-8. [Crossref] [PubMed]
- Cordenonsi M, Zanconato F, Azzolin L, et al. The hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 2011;147:759-72. [Crossref] [PubMed]
- Nusse R, Fuerer C, Ching W, et al. Wnt signaling and stem cell control. Cold Spring Harb Symp Quant Biol 2008;73:59-66. [Crossref] [PubMed]
- Tepera SB. A beta-catenin survival signal is required for normal lobular development in the mammary gland. J Cell Sci 2003;116:1137-49. [Crossref] [PubMed]
- Li Y, Welm B, Podsypanina K, et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc Natl Acad Sci U S A 2003;100:15853-8. [Crossref] [PubMed]
- Liu BY, McDermott SP, Khwaja SS, et al. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci U S A 2004;101:4158-63. [Crossref] [PubMed]
- Kameda C, Tanaka H, Yamasaki A, et al. The Hedgehog pathway is a possible therapeutic target for patients with estrogen receptor-negative breast cancer. Anticancer Res 2009;29:871-9. [PubMed]
- Koga K, Nakamura M, Nakashima H, et al. Novel link between estrogen receptor alpha and hedgehog pathway in breast cancer. Anticancer Res 2008;28:731-40. [PubMed]
- Lee MY, Sun L, Veltmaat JM. Hedgehog and gli signaling in embryonic mammary gland development. J Mammary Gland Biol Neoplasia 2013;18:133-8. [Crossref] [PubMed]
- Visbal AP, LaMarca HL, Villanueva H, et al. Altered differentiation and paracrine stimulation of mammary epithelial cell proliferation by conditionally activated Smoothened. Dev Biol 2011;352:116-27. [Crossref] [PubMed]
- Zhang X, Harrington N, Moraes RC, et al. Cyclopamine inhibition of human breast cancer cell growth independent of Smoothened (Smo). Breast Cancer Res Treat 2009;115:505-21. [Crossref] [PubMed]
- Jeng KS, Sheen IS, Jeng WJ, et al. High expression of Sonic Hedgehog signaling pathway genes indicates a risk of recurrence of breast carcinoma. Onco Targets Ther 2013;7:79-86. [Crossref] [PubMed]
- García-Zaragoza E, Pérez-Tavarez R, Ballester A, et al. Intraepithelial paracrine Hedgehog signaling induces the expansion of ciliated cells that express diverse progenitor cell markers in the basal epithelium of the mouse mammary gland. Dev Biol 2012;372:28-44. [Crossref] [PubMed]
- Fiaschi M, Rozell B, Bergström A, et al. Development of mammary tumors by conditional expression of GLI1. Cancer Res 2009;69:4810-7. [Crossref] [PubMed]
- Zhao J. Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacol Ther 2016;160:145-58. [Crossref] [PubMed]
- Borst P, Jonkers J, Rottenberg S. What makes tumors multidrug resistant? Cell Cycle 2007;6:2782-7. [Crossref] [PubMed]
- Yoshida GJ, Saya H. Therapeutic strategies targeting cancer stem cells. Cancer Sci 2016;107:5-11. [Crossref] [PubMed]
- Osuka S, Sampetrean O, Shimizu T, et al. IGF1 receptor signaling regulates adaptive radioprotection in glioma stem cells. Stem Cells 2013;31:627-40. [Crossref] [PubMed]
- Lv X, Wang Y, Song Y, et al. Association between ALDH1+/CD133+stem-like cells and tumor angiogenesis in invasive ductal breast carcinoma. Oncol Lett 2016;11:1750-6. [Crossref] [PubMed]
- Stacy AE, Jansson PJ, Richardson DR. Molecular Pharmacology of ABCG2 and Its Role in Chemoresistance. Mol Pharmacol 2013;84:655-69. [Crossref] [PubMed]
- Cojoc M, Mäbert K, Muders MH, et al. A role for cancer stem cells in therapy resistance: Cellular and molecular mechanisms. Semin Cancer Biol 2015;31:16-27. [Crossref] [PubMed]
- Naor D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res 1997;71:241-319. [Crossref] [PubMed]
- Thapa R, Wilson GD. The Importance of CD44 as a Stem Cell Biomarker and Therapeutic Target in Cancer. Stem Cells Int 2016;2016. [Crossref] [PubMed]
- Charrad RS, Li Y, Delpech B, et al. Ligation of the CD44 adhesion molecule reverses blockage of differentiation in human acute myeloid leukemia. Nat Med 1999;5:669-76. [Crossref] [PubMed]
- Smith LM, Nesterova A, Ryan MC, et al. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer 2008;99:100-9. [Crossref] [PubMed]
- Zhao L, Yang Y, Zhou P, et al. Targeting CD133high Colorectal Cancer Cells In Vitro and In Vivo With an Asymmetric Bispecific Antibody. J Immunother 2015;38:217-28. [Crossref] [PubMed]
- Annett S, Robson T. Targeting cancer stem cells in the clinic: Current status and perspectives. Pharmacol Ther 2018;187:13-30. [Crossref] [PubMed]
- Smalley M, Piggott L, Clarkson R. Breast cancer stem cells: Obstacles to therapy. Cancer Lett 2013;338:57-62. [Crossref] [PubMed]
- Zang S, Chen F, Dai J, et al. RNAi-mediated knockdown of Notch-1 leads to cell growth inhibition and enhanced chemosensitivity in human breast cancer. Oncol Rep 2010;23:893-9. [PubMed]
- Suman S, Das TP, Damodaran C. Silencing NOTCH signaling causes growth arrest in both breast cancer stem cells and breast cancer cells. Br J Cancer 2013;109:2587-96. [Crossref] [PubMed]
- Hao J, Zhang Y, Wang Y, et al. Role of extracellular matrix and YAP/TAZ in cell fate determination. Cell Signal 2014;26:186-91. [Crossref] [PubMed]
- Gupta S, Takebe N, Lorusso P. Targeting the Hedgehog pathway in cancer. Ther Adv Med Oncol 2010;2:237-50. [Crossref] [PubMed]
- Zhao C, Chen A, Jamieson CH, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature 2009;458:776-9. [Crossref] [PubMed]
- Berman DM, Karhadkar SS, Hallahan AR, et al. Medulloblastoma growth inhibition by Hedgehog pathway blockade. Science 2002;297:1559-61. [Crossref] [PubMed]
- Thayer SP, Di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature 2003;425:851-6. [Crossref] [PubMed]
- Takebe N, Miele L, Harris PJ, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat Rev Clin Oncol 2015;12:445-64. [Crossref] [PubMed]
- Moon CM, Kwon JH, Kim JS, et al. Nonsteroidal anti-inflammatory drugs suppress cancer stem cells via inhibiting PTGS2 (cyclooxygenase 2) and NOTCH/HES1 and activating PPARG in colorectal cancer. Int J Cancer 2014;134:519-29. [Crossref] [PubMed]
- Gurney A, Axelrod F, Bond CJ, et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci 2012;109:11717-22. [Crossref] [PubMed]
- Kahn M. Can we safely target the WNT pathway? Nat Rev Drug Discov 2014;13:513-32. [Crossref] [PubMed]
- Trikha M, Corringham R, Klein B, et al. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res 2003;9:4653-65. [PubMed]
- Guo Y, Xu F, Lu T, et al. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev 2012;38:904-10. [Crossref] [PubMed]
- Ginestier C, Liu S, Diebel ME, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest 2010;120:485-97. [Crossref] [PubMed]
- Mohammadpour F, Ostad SN, Aliebrahimi S, et al. Anti-invasion Effects of Cannabinoids Agonist and Antagonist on Human Breast Cancer Stem Cells. Iran J Pharm Res 2017;16:1479-86. [PubMed]
Cite this article as: Saeg F, Anbalagan M. Breast cancer stem cells and the challenges of eradication: a review of novel therapies. Stem Cell Investig 2018;5:39.


