Haemostatic potential of human bone marrow-derived mesenchymal stromal cells in Wistar rats with carbon tetrachloride induced liver cirrhosis
Introduction
In the bone marrow, there are many populations of stem cells including hematopoietic stem cells, mesenchymal stem cells, and multipotent adult progenitor cells. Bone marrow-derived mesenchymal stromal cells (BM-MSCs) are involved in many key events related to hematopoiesis, immune cell generation and activation, immunomodulation, and immune tolerance (1). Due to these characteristics, BM-MSCs have been widely used in clinical trials and treatments (2). BM-MSCs have received wider attention because they can be easily isolated from a small aspirate of bone marrow and can be expanded in culture and characterized in vitro. MSCs also offer several other advantages like long-term storage without significant loss of potency and no adverse reactions to allogeneic MSCs transplant (3).
Acute liver failure is a life-threatening condition caused by variety of etiologies including drugs, viral infections, alcohol, metabolic, autoimmune, or genetic disorders which may cause acute hepatic dysfunction leading to liver failure (4). The liver plays an important role in the maintenance of haemostasis as it is the site of synthesis for most of proteins required for regulation of coagulation and fibrinolysis (5). Some of the proteins synthesized by the liver include coagulation factors I (fibrinogen), II (prothrombin), V, VII, VIII, IX, X, XI, XIII. Thus, injury of the liver parenchymal cells can disturb haemostasis resulting in the development of multiple coagulation abnormalities. Patients with chronic liver disease will have significantly reduced levels of these factors which indicates extensive hepatocellular damage. CCl4 is a selective hepatotoxic chemical agent which triggers lipid and protein peroxidation with consequent death of hepatocytes. Many investigators have utilized this chemical to produce liver cirrhosis in experimental animals (6).
The involvement of MSCs in the wound-healing process and their role in the coagulation cascade has been suggested by various observations (7) but has not been formally proved. Nevertheless, no attempt has been made to come to a logical conclusion about the beneficial effects of MSCs on haemostasis. In view of the above lacunae, the present study was designed to investigate the haemostatic potential of BM-MSCs in vivo. We have used silymarin in our study as a reference drug to compare the beneficial effects achieved by BM-MSCs as silymarin is the most well-researched plant product in the treatment of liver disease. The aim of the present study was to evaluate the effect of BM-MSCs and the combination of BM-MSCs and silymarin on the prothrombin time and fibrinogen concentration in experimentally induced rat model of liver cirrhosis. In addition, the therapeutic effect of BM-MSCs was studied using liver enzyme level analysis, histopathology and scanning electron microscopy (SEM) study.
Methods
Drugs and reagents
Silymarin was procured from Sigma Chemical Inc. (USA), Carbon tetrachloride (CCl4) and all other chemicals were obtained from Merck Chemicals, Mumbai (India). All reagents were of analytical grade, stored in a refrigerator at +4 °C and were equilibrated at room temperature before the start of analysis.
Animals
Female Wistar albino rats (4–5 months old), weighing 140–160 g was chosen for this study. Animals were bred in the central animal house of MAHE, Manipal and were housed in polypropylene cages, maintained under standard conditions with temperature (26–30 °C), 12-h light/12-h dark cycle and relative humidity of 40–60%. Rats had continuous access to tap water and regular calorie standard rat pellet diet (VRK’s Scientist’s Choice Laboratory animal feed, VRK nutritional solutions, Maharashtra, India). The rats were adapted to the laboratory conditions for one week before the start of the experiment. The experiment was done after obtaining approval from the Institutional Animal Ethics Committee (IAEC/KMC/20/2014) and conducted in accordance with the ethical norms of the Ministry of Social Justices and Empowerment, Government of India and Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA) guidelines.
Isolation and culture of BM-MSCs
Bone marrow aspirates from healthy adult volunteers were obtained after informed consent and under institutional ethical rules. BM-MSCs were isolated and expanded using the method of Pal et al. (8). Freshly-thawed BM-MSCs was used for intravenous administration into the rats.
Experimental design
This was an experimental study. Sixty rats were randomly divided into six groups containing ten rats in each group.
CCl4 induced liver cirrhosis model
Rats were injected with CCL4 (1 mL/kg of body weight) thrice weekly for 28 days intraperitoneally (IP).
Experimental groups
Group 1: (normal control group) ten rats which did not receive CCl4. Fifty rats were injected with CCl4 to induce liver cirrhosis as scheduled above and then divided into following groups. Group 2: (CCl4 trt group) which served as disease model and injected with CCl4 only. Group 3: (CCl4 + low dose BM-MSCs group) injected with single dose of 3.25 million humans equivalent dose of BM-MSCs/kg body wt, intravenously. Group 4: (CCl4 + high dose BM-MSCs group) injected with single dose of 9.75 million humans equivalent dose of BM-MSCs/kg body wt, intravenously. Group 5: (CCl4 + silymarin group) received silymarin at the dose of 100 mL/kg body wt, orally. Group 6: (CCl4 + silymarin + BM-MSCs group), received silymarin (100 mL/kg body wt) and high dose BM-MSCs (9.75 million MSCs/kg body wt). The cell number used in this study was based on dose optimization performed in a pilot study (9). The BM-MSCs and silymarin treatment was given after 28 days of CCl4 intoxication. BM-MSCs were formulated in 0.5 mL Plasma-Lyte A and injected intravenously into the tail vein of the rat after the disease model was confirmed by histopathology. Cells were administered slowly and cautiously to avoid embolism. The entire study period was sixty days. At the end of the experiment period, the rats were anesthetized, and blood samples were collected from the retro-orbital venous plexus. After blood collection, the animals were sacrificed by administering overdose of ketamine, dissected and the liver samples from each rat was rapidly excised, washed thoroughly with isotonic saline and processed for histopathological and SEM study.
Liver enzyme analysis
On the 30th day after treatments, the animals were anesthetized with ketamine (80 mg/kg; IP) following a 12 hrs fast. Blood was collected in small centrifuge tubes by orbital puncture and was allowed to clot. It was then centrifuged for 10 minutes at 3,000 rpm. Serum was separated and used immediately for liver enzyme estimations. Alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST) enzyme levels in serum were estimated using commercially available kits obtained from Agappe Diagnostics Ltd.
Estimation of prothrombin time
Prothrombin time was estimated using the kit obtained from Agappe Diagnostics Ltd. Blood was collected in small centrifuge tubes containing 3.2% of sodium citrate. The sample was then centrifuged at 3,000 rpm for 15 min to obtain plasma. Test was performed according to the instructions given in the kit. Briefly, 0.1 mL of test plasma was placed in the water bath at 37 °C. 0.2 mL of pre-warmed thromboplastin reagent was added to the test sample, and stopwatch was started. The time taken for clot to form was noted.
Determination of fibrinogen concentration
Plasma fibrinogen concentration was determined according to the clot weight method of Ingram (1961) with modifications. Blood was collected in small centrifuge tubes containing 3.2% of sodium citrate. The sample was then centrifuged at 3,000 rpm for 15 min to obtain plasma. Zero point two mL of the test sample was taken in a test tube and incubated for 2 min at 37 °C. In total, 100 µL of 0.025 M CaCl2 and 0.2 mL of thrombin reagent obtained from r2 Hemostasis Diagnostics India Pvt. Ltd, was added to test sample, mixed and the resulting clot which was formed was harvested with a small wooden stick. The clot was then transferred into a small bottle containing acetone to dry and harden for about 10 min; the acetone was emptied and the clot was placed on a filter paper for the remaining acetone to evaporate. The clot was then recovered and weighed. Thus, fibrinogen concentration of citrated plasma in mg/dL equals clot weight (mg) divided by plasma volume (dL).
Histopathological examination
The liver tissue samples from the rats of each group was washed, fixed in 10% formalin and dehydrated in ascending grades of alcohol. It was then embedded in paraffin. 24 hours after block preparation, paraffin sections were obtained on clean glass slides using microtome. The sections were stained with hematoxylin and eosin (H&E) stain and observed for histopathological changes under a light microscope. Relevant photomicrographs were taken.
SEM study
Liver tissue blocks from the animals of all the groups were prepared and processed for SEM for a 3D-architectural study. Each of the liver tissue samples (2–3 mm thick) was gently washed in 0.5 M potassium phosphate buffer, at pH 7.2, and 4 °C. Sections were fixed in 2.5% glutaraldehyde solution at 4 °C for 6–8 hours. The sample was washed with 0.1 M phosphate buffered saline (PBS) for 15 min. A series of ascending grades of ethyl alcohol were used for 15 min each to dehydrate the specimens. Specimens were then air dried, coded and mounted on aluminum stubs. Liver sections were viewed at various magnifications on Zeiss Evo 18 scanning electron microscope (Carl Zeiss Microscopy, LLC, Thornwood, NY, USA) to evaluate the surface morphology. Photographs were taken from randomly selected fields.
Statistical analysis
Results were analyzed using Statistical Package for the Social Sciences (SPSS version 16.0). Data were expressed as mean ± standard deviation and analyzed by one-way analysis of variance (ANOVA), followed by post hoc Tukey’s test. P<0.05 was considered statistically significant.
Results
Effect of BM-MSCs on liver enzyme levels
In CCl4 treated rats, serum levels of AST, ALT, ALP enzymes were significantly increased when compared to the normal control group (P<0.001) indicating the injury caused by CCl4 intoxication. This also confirmed the liver cirrhosis disease model. Further, it was observed that the treatment with two doses of BM-MSCs, silymarin and the combination treatment of silymarin + BM-MSCs was effective in decreasing the liver enzyme levels when compared to the CCl4 treated group (P<0.001). It was also observed that the combination treatment of silymarin and BM-MSCs significantly decreased the liver enzyme levels when compared with other treatments (P<0.01) (Table 1). These results showed that BM-MSC transplantation facilitated liver function recovery.
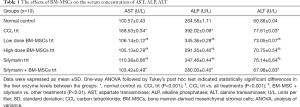
Full table
Effect of BM-MSCs on prothrombin time
The mean prothrombin time in the normal control group was 27.0±1.1 seconds. Significant increase in prothrombin time was seen in CCl4 treated group when compared to normal control group (P<0.001). Treatment with BM-MSCs, silymarin, and combination of BM-MSCs and silymarin significantly reversed CCl4 induced alterations in the prothrombin time when compared with the CCl4 trt group (P<0.001). The combination of BM-MSCs and silymarin treatment showed a better reduction in the prothrombin time compared to other treatments (P<0.01), except high dose BM-MSCs treatment (P>0.05) (Table 2).
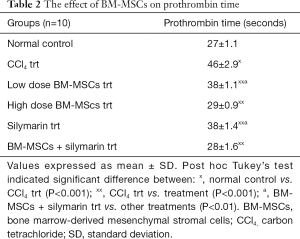
Full table
Effect of BM-MSCs on plasma fibrinogen concentration
The mean plasma fibrinogen level in the normal control group was 122.62±2.3 mg/dL. Significant decrease in plasma fibrinogen concentration was seen in CCl4 treated group when compared to normal control group (P<0.001). Treatment with BM-MSCs, silymarin, and combination of BM-MSCs and silymarin significantly increased the plasma fibrinogen concentration when related with the CCl4 trt group (P<0.001). The combination of BM-MSCs and silymarin treatment showed a significant change in fibrinogen concentration compared to other treatments (P<0.001) (Table 3).
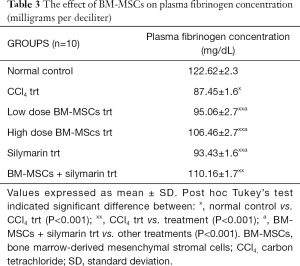
Full table
Histopathology study
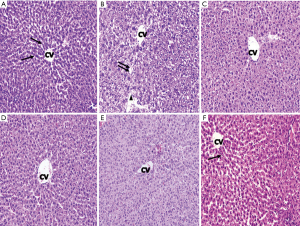
Histopathological investigation of liver sections of the normal control group showed distinct hepatocytes radiating from the central vein with narrow sinusoidal spaces. Liver sections of CCl4 intoxicated animals showed disarray of hepatic cells with necrosis, fatty degeneration, vacuolization, homogeneous cytoplasm of the hepatocytes was observed, which confirmed the successful establishment of a rat model of liver cirrhosis. The liver sections of the rats treated with low and high dose of BM-MSCs succeeding CCl4 intoxication showed positive changes which was depicted by the absence of necrosis and vacuoles. However, small amount of fibrous tissue, wider sinusoidal spaces were observed. Treatment with silymarin did not show notable reduction in the fibrosis, but moderately reduced the inflammatory activity. Liver sections of combination treatment of BM-MSCs and silymarin showed normal and distinct hepatocytes arranged as hepatic cords, with no vacuoles and reduced sinusoidal spaces. This appearance indicated the effectiveness of combination treatment of high dose BM-MSCs and silymarin in protecting the liver cells against CCl4 induced liver damage (Figure 1).
SEM study
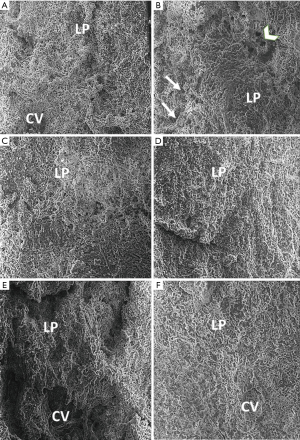
Scanning electron micrograph of the liver of normal control group rats showed the hepatocytes with the regular smooth surface, separated by narrow blood sinusoids, with a negligible amount of fibrous tissue. Electron micrographs of the CCl4 treated toxic group showed that the liver had an irregular surface with most of the hepatocytes appearing necrotic. Few hepatocytes seemed necrotic with dilated blood sinusoids. Many cyst-like structures, looking like a honeycomb, were found surrounded by fibers, which were the pseudo lobules. The network of fibers formed this lobule. Other pathological features observed were the destruction of the lobular architecture, inflammation, foamy vacuolated cytoplasm. Profuse collagen fiber deposits were found to fill numerous areas in the extracellular spaces of the liver parenchyma. Scanning electron micrograph of the low dose BM-MSCs, high dose BM-MSCs, and silymarin treatment showed that most of the hepatocytes appeared preserved; however, some hepatocytes still appeared degenerated, and the irregular surface was seen. The sinusoid spaces had reduced and appeared preserved. However, few strands of collagen fiber bands were found among the hepatocytes.
Treatment with the combination of silymarin and high dose BM-MSCs markedly reduced the amount of collagen fiber bands, and the morphology of the hepatocytes was comparable to that of the normal control group with distinct hepatocytes and narrow sinusoidal spaces. The liver surface morphology appeared smooth (Figure 2).
Discussion
Haemostasis is the first phase of wound healing which involves three successive steps: vasoconstriction, blocking the wound by platelet aggregation and blood coagulation (10). Normal haemostasis results from an interaction between coagulation factors (pro and anticoagulants), fibrinolytics and platelets. Recent literature indicates that BM-MSCs secrete a variety of factors that help repair of tissues, stimulate differentiation and proliferation of endogenous tissue progenitors, and reduce inflammatory and immune reactions (11,12). Herein, we have evaluated the potential of BM-MSCs and its combination with silymarin on the haemostatic mechanism, with prime attention on how it affects prothrombin time and plasma fibrinogen concentration in a liver cirrhosis animal model.
Developing an animal model representing the same complexity of human diseases is crucial in an experimental study. In this work, we induced liver cirrhosis in rats by CCl4 intoxication. CCl4 is one of the fastest, easiest, and reliable methods to develop cirrhosis and can be used to screen anti-fibrotic agents. CCl4 gets accumulated in the hepatocytes and leads to formation of free radicals and subsequently lipid peroxidation. The lipid peroxidation produces breakdown of the bio-membrane at cellular and sub-cellular levels leading to hepatocyte destruction (13). Liver enzyme level analysis and histopathology study performed in the present study confirmed the cirrhosis model. Histopathology and SEM results indicated vacuole formation, necrosis, and disarrangement of hepatocytes in liver sections treated with CCl4. Liver tissue of CCl4 intoxicated rats treated with a combination of BM-MSCs and silymarin exhibited hepatoprotective activity with the disappearance of fatty changes and necrosis which was comparable with the normal control liver sections.
Collagen turnover and extracellular matrix (ECM) remodeling are regulated by various matrix metalloproteinases (MMPs) and their inhibitors, i.e., the tissue inhibitors of metalloproteinases (TIMPs). MMPs and TIMPs are crucial for matrix remodeling processes during hepatic fibrogenesis. During spontaneous recovery from liver fibrosis, there is a decrease in TIMP expression, an increase in collagenase activity, and increase in apoptosis of hepatic stellate cells (14). In several fibrosis models, MSCs have been shown to increase the expression of MMPs (15) or to decrease TIMP-1 expression (16), and these alterations are associated with fibrosis resolution. BM-MSCs suppress the pathophysiological process that is mediated by chronic inflammation and contributes to a modification of the microenvironment; the result is diminished tissue fibrosis, increased resident stem cell proliferation and eventually tissue regeneration.
The liver is the chief site for synthesis of most of proteins that play a central role in sustaining hemostasis. Thus, injury of liver parenchymal cell function due to fibrosis and cirrhosis can disturb haemostasis and blood coagulation. Cirrhosis is associated with increased risk of bleeding as all coagulation factors are reduced in this condition except factor VIII. Prothrombin time and fibrinogen concentration are indices for the measurement of blood coagulation. While the prothrombin time measures the extrinsic pathway in blood coagulation, fibrinogen level is critical for the formation of a firm fibrin clot (17). Prothrombin test is a screening test for the extrinsic coagulation system, that is, factor VII and it can also detect deficiencies of factor V, X, prothrombin, and fibrinogen (18). Previous studies indicate that BM-MSCs secrete soluble factors and thereby rejuvenate or repair diseased cells and tissues (19). Such biofactors secreted by BM-MSCs play a significant role in various aspects of hematopoiesis and have been named as “trophic factors” (20). In this study, it was seen that BM-MSCs and its combination treatment with silymarin significantly decreased the prothrombin time in disease models. This may be because of the trophic factors released by BM-MSCs, which lead to increase in the prothrombin concentration or increase in the level of one of the other extrinsic coagulation factors.
Fibrinogen is a glycoprotein present in the plasma, synthesized in the liver (21). Neubauer et al. have reported that fibrin deposits around the necrotic central vein and within fibrotic septa after CCl4 induced liver damage (22). Decrease in fibrinogen levels is used as a biomarker for initiation of the coagulation system and as a monitor of anticoagulant efficiency. CCl4 treated animals showed significant reduction in plasma fibrinogen levels which might be due to the increase of fibrinogen degrading products. BM-MSCs and its combination with treatment with silymarin showed significant rise in the plasma fibrinogen levels comparable to normal control animals. This effect may be due to the fact that BM-MSCs secrete many growth factors and cytokines, which shows anti-apoptotic activity in hepatocytes (23), regulate cellular responses, angiogenesis formation and tissue remodeling (24) and play a vital role in wound-healing process including haemostasis.
In recent years, there has been significant progress in the understanding of the pathophysiologic basis of common haematological disorders in the background of liver disease. Results of this study suggest that BM-MSCs and their synergistic effect with silymarin may represent a novel therapeutic strategy in regenerative medicine and for treating bleeding disorders. However, the mechanisms mediating these events and exactly how BM-MSCs contribute in haemostasis remain undefined. Investigations are still needed in this regard, to reveal the molecular bases behind such observations.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The experiment was done after obtaining approval from the Institutional Animal Ethics Committee (IAEC/KMC/20/2014) and conducted in accordance with the ethical norms of the Ministry of Social Justices and Empowerment, Government of India and Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA) guidelines.
References
- Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell 1997;88:287-98. [Crossref] [PubMed]
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem 1997;64:278-94. [Crossref] [PubMed]
- Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng 2010;12:87-117. [Crossref] [PubMed]
- Polson J, Lee WM. AASLD position paper: The management of acute liver failure. Hepatology 2005;41:1179-97. [Crossref] [PubMed]
- Amitrano L, Guardascione MA, Brancaccio V, et al. Coagulation disorders in liver disease. Semin Liver Dis 2002;22:83-96. [Crossref] [PubMed]
- Parola M, Leonarduzzi G, Biasi F, et al. Vitamin E dietary supplementation protects against carbon tetrachloride-induced chronic liver damage and cirrhosis. Hepatology 1992;16:1014-21. [Crossref] [PubMed]
- Maxson S, Lopez EA, Yoo D, et al. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 2012;1:142-9. [Crossref] [PubMed]
- Pal R, Hanwate M, Jan M, et al. Phenotypic and functional comparison of optimum culture conditions for upscaling of bone marrow-derived mesenchymal stem cells. J Tissue Eng Regen Med 2009;3:163-74. [Crossref] [PubMed]
- Aithal AP, Bairy LK, Seetharam RN. Safety assessment of human bone marrow-derived mesenchymal stromal cells transplantation in Wistar rats. J Clin Diagn Res 2017;11:FF01-3. [PubMed]
- Al-Shaibani MB, Wang X, Lovat PE, et al. Cellular Therapy for Wounds: Applications of Mesenchymal Stem Cells in Wound Healing. In: Alexandrescu A. editor. Wound Healing—New insights into Ancient Challenges. London: InTech, 2016.
- Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99:3838-43. [Crossref] [PubMed]
- Raffaghello L, Bianchi G, Bertolotto M, et al. Human Mesenchymal Stem Cells Inhibit Neutrophil Apoptosis: A Model for Neutrophil Preservation in the Bone Marrow Niche. Stem cells 2008;26:151-62. [Crossref] [PubMed]
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815-22. [Crossref] [PubMed]
- Liu CH, Hwang SM. Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine 2005;32:270-9. [Crossref] [PubMed]
- Wu Y, Huang S, Enhe J, et al. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. Int Wound J 2014;11:701-10. [Crossref] [PubMed]
- Ali G, Mohsin S, Khan M, et al. Nitric oxide augments mesenchymal stem cell ability to repair liver fibrosis. J Transl Med 2012;10:75. [Crossref] [PubMed]
- Ryeom HK, Kim SH, Kim JY, et al. Quantitative Evaluation of Liver Function with MRI Using Gd-EOB-DTPA. Korean J Radiol 2004;5:231-9. [Crossref] [PubMed]
- Ochei J, Kolhatkar A. Medical Laboratory Science. Theory and Practice. New Delhi: Tata Mcgraw-Hill Publishing Company Limited, 2000:331-49.
- Monica C. District laboratory practice in tropical countries part 2. Cambridge: Cambridge University Press, 2000:207-66.
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076-84. [Crossref] [PubMed]
- Majumdar MK, Thiede MA, Haynesworth SE, et al. Human marrow derived-mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res 2000;9:841-8. [Crossref] [PubMed]
- Neubauer K, Knittel T, Armbrust T, et al. Accumulation and cellular localization of fibrinogen/ fibrin during short- term and long- term rat liver injury. Gastroenterology 1995;108:1124-35. [Crossref] [PubMed]
- Trim N, Morgan S, Evans M, et al. Hepatic stellate cells express the low affinity nerve growth factor receptor p75 and undergo apoptosis in response to nerve growth factor stimulation. Am J Pathol 2000;156:1235-43. [Crossref] [PubMed]
- Hocking AM, Gibran NS. Mesenchymal stem cells: paracrine signaling and differentiation during cutaneous wound repair. Exp Cell Res 2010;316:2213-19. [Crossref] [PubMed]
Cite this article as: Aithal AP, Bairy LK, Seetharam RN, Kumar N. Haemostatic potential of human bone marrow-derived mesenchymal stromal cells in Wistar rats with carbon tetrachloride induced liver cirrhosis. Stem Cell Investig 2018;5:21.


